

Review - DOI:10.33594/000000612
Accepted 23 February, 2023 - Published online : 15 March 2023
1Center for Physiology, Pathophysiology and Biophysics, Institute for Physiology and Pathophysiology, Salzburg, Austria;
2Department of Internal Medicine I, University Hospital Salzburg, Salzburger Landeskliniken, Salzburg, Austria;
3Gastein Research Institute, Paracelsus Medical University, Salzburg, Austria;
4Institute of Nursing Science and Practice, Paracelsus Medical University, Salzburg, Austria;
5Institute of Anatomy and Cell Biology, Paracelsus Medical University, Salzburg, Austria;
6Ludwig Boltzmann Institute for Arthritis and Rehabilitation, Paracelsus Medical University, Salzburg, Austria;
7Chondrometrics GmbH, Freilassing, Germany;
8Kathmandu University School of Medical Sciences, Dhulikhel, Nepal
Osteoarthritis (OA) is a major cause of pain and disability in adults, affecting approximately 150 million people worldwide. It is most prevalent in knees and hips. Major risk factors are age, female sex, prior joint injury, and obesity. OA causes significant personal and steeply rising socio-economical costs in ageing populations. OA is characterized by progressive cartilage damage and inflammation. In later stages, it affects the subchondral bone, bone marrow, ligaments, tendons, and nerves und eventually leads to joint failure. Symptoms include pain, joint swelling, and stiffness. Therapies are symptomatic and focus on pain relief and measures to improve mobility, or, ultimately, joint replacement. So far, no drugs that could prevent or slow down disease progression are available. Based on promising in vitro and preclinical studies, recombinant fibroblast growth factor (FGF) 18 (sprifermin; Merck Serono) has come into focus as a potential disease-modifying OA drug (DMOAD). Three randomized controlled trials (RCTs) investigating the safety and efficacy of intraarticularly (i.a.) injected sprifermin application in patients with knee OA have been completed so far. Data from these trials, post hocanalyses and follow-ups provide have evidenced that i.a. sprifermin induced a significant and sustained increase in cartilage thickness and volume without specific adverse effects, but in terms of clinical symptoms or physical joint function sprifermin did not cause significant improvements compared to placebo treatment in whole study populations. However, significant pain reduction was observed in a “subgroup at risk” of patients with more severe disease states, indicating that under certain disease conditions the structural benefit improvements could translate into clinical benefit. This calls for larger RCTs allowing e.g., for disease state or risk factor-specific stratification of patients and longer follow-ups to substantiate the efficacy of sprifermin as a possible DMOAD. This review gives an overview on the prevalence, etiology and socio-economic burden of OA, its pathogenesis as well as the current treatment options of the disease. It summarizes the role of FGF-18 in chondrocyte and cartilage (patho)physiology and addresses the question of evidence for the efficacy and safety of i.a. sprifermin injection in patients with knee OA based on trial outcomes and literature data.
Prevalence and Etiology of OA
In general, arthritis refers to more than 100 types of conditions associated with joint pain and disease such as osteoarthritis, rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, osteoporosis, or fibromyalgia [1]. Arthritis may affect single or multiple joints. In most parts of the world, arthritis is the leading cause of disability, impaired mobility, and reduction in the Quality of Life (QoL). It affects people of all sexes, ages, and ethnics [1]. In the USA, one in four adults aged 18–64 years are estimated to have arthritis, resulting in a prevalence of approximately 91 million people with physician-diagnosed arthritis and/or symptoms consistent with arthritis [2]. According to the Centers for Disease Control and Prevention (CDC), an estimated 22.7% (~54 million) of US adults had physician-diagnosed arthritis by 2015, and the prevalence is expected to increase in the next decades to ~78 millions in 2040 [3] (Fig. 1). In Austria, according to the 2019 Health Survey on chronic diseases, on average 17.7% of women and 10.7% of men were suffering from arthritis dependent on age, with a 12-month prevalence of ~18% and ~13% in women and men aged 45–59 years, respectively, and ~47% and ~28% aged > 75 years. In people aged 60–74 years, one in three women and one in five men were affected according to the Austrian Health Survey 2019 [4].
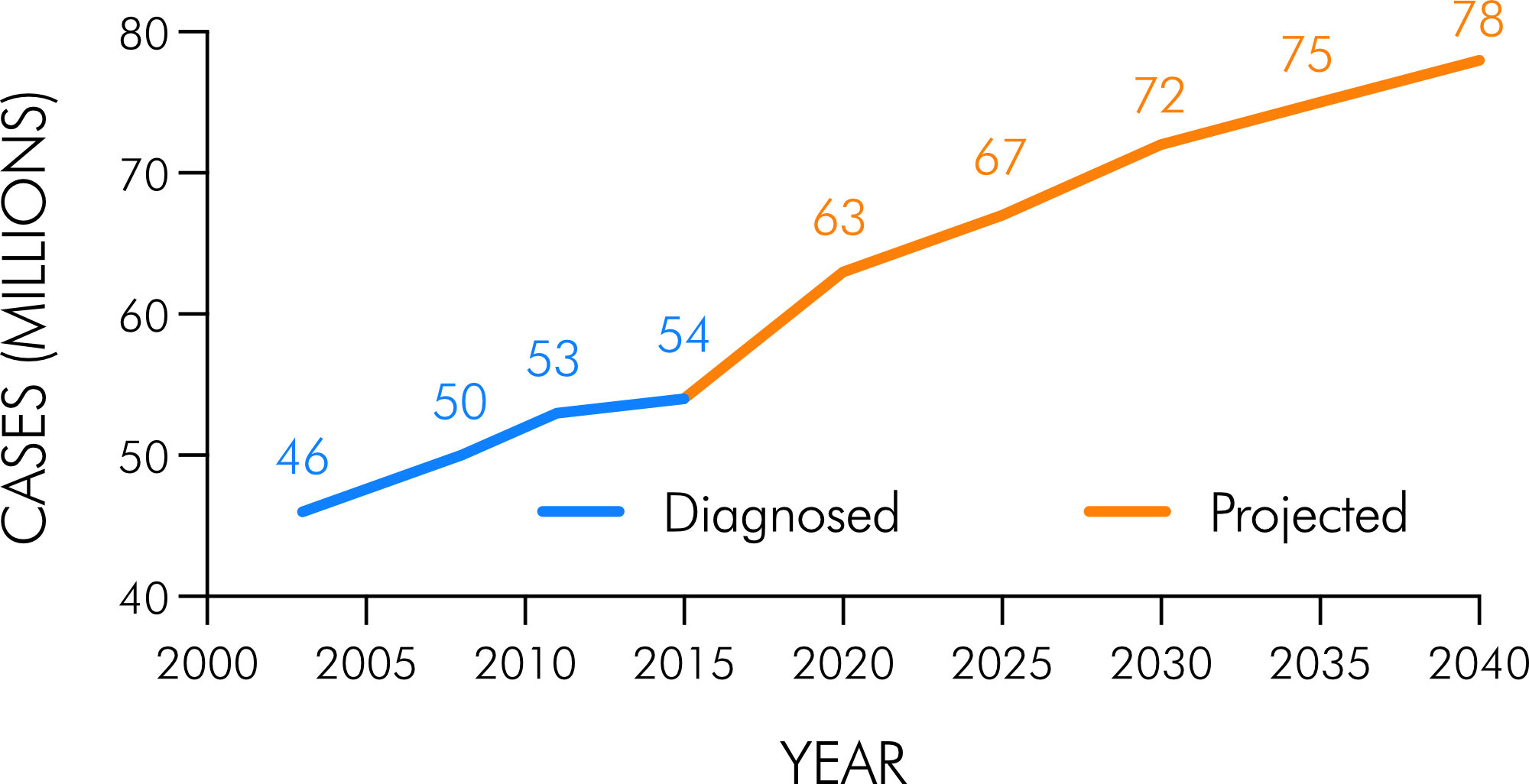
Figure 1. Physician-diagnosed and future projections of arthritis in the adult US population (aged 18 years). Graph created from data in [3].
Osteoarthritis (OA) is the most prevalent form of arthritis and one of the most common forms of musculoskeletal diseases, and a major cause of pain and disability in adults, currently affecting approximately 150 million people worldwide. The incidence increases with age: by an age of 65, ~80% have radiographic evidence of OA. The disease affects the articular cartilage of diarthrodial joints and adjacent tissues such as subchondral bone, bone marrow and the synovial membrane, and ultimately leads to joint destruction. It occurs most often in knees and hips (gonarthrosis and coxarthrosis, respectively) accounting for a large proportion of the OA burden, but may also affect the neck, the lower back and small joints of fingers and toes [1, 5-9].
OA is classified as primary or secondary [6]. Primary or idiopathic OA is more common and also referred to as “wear and tear” OA, as it develops with age due to joint usage. Secondary OA has one or more specific causes such as an injury (post-traumatic OA), lifestyle (inactivity), joint misalignment, surgical intervention (post-operative OA), obesity, metabolic diseases, genetic predisposition, endocrine factors (e.g., post-menopausal changes), or inflammation from other diseases (e.g., rheumatoid arthritis) [6]. Most people develop primary OA with increasing age, but a significant number of younger patients develop OA as the result of excess or abnormal mechanical loading of joints. There are age and sex-dependent differences in the OA prevalence: below 45 years it is more prevalent in men, while over 45 years it is more prevalent among females [1, 5, 7-11]. Major risk factors to develop OA are age, female sex, prior joint injury and obesity (Body Mass Index > 30 kg/m2) [2, 5, 6, 8, 11-13].
Based on data of the 2017 Global Burden of Disease (GBD), Injuries and Risk Factors study led by the Institute of Health Metrics and Evaluation [14], which comprises data from 195 countries and territories, Safiri et al. reported on global-, regional- and national-level prevalence, incidence and percentages change in prevalence of hip and knee OA between 1990 and 2017 [9]. The left panel of Fig. 2 shows the age-adjusted OA prevalence per 100, 000 population in 2017 for both sexes on the global level, in high-income North America and parts of Europe. Globally, the prevalence was ~3, 800 per 100, 000 inhabitants in 2017 (~300, 000, 000 people in total worldwide) with the highest number of cases in North America (~6, 100) and ~3, 200–3, 900 cases in Europe. Compared to 1990, this was an increase of over 9% globally, 7.2–9.3% in Europe and 22.5% in North America (Fig. 2, right panel). The global age-standardized incidence per 100, 000 population in 2017 was ~180 (~306.6 in North America and 160.9–200.8 in Europe) [9]. Only in the USA, in 2008 the number of people affected by OA was estimated on 27 million adults up from 21 million in 1995 [15] and a decade later, the Osteoarthritis Action Alliance and the Arthritis Foundation reported on 32.5 million US adults having physician-diagnosed OA [1, 7], referring to data from the US Bone and Joint Initiative [16]. In Austria, based on the analysis of micro-census data of the Austrian Health Interview Survey (AT-HIS) 2006–07, the prevalence of OA was estimated to be ~12% in men and ~19% in women over 15 years [10]. We have recently estimated the expected number of OA patients in the future, which revealed an overall increase in the total number of patients from 2019 to 2080 of 38% for men and women. The most affected groups are those aged 70–79 and 80+. The increases based on an assumed main scenario (mean fertility, rate of immigration and life expectancy) are forecasted to be 45% and 245%, respectively, for men and 28% and 148%, respectively, for women. Assuming a more plausible population growth scenario (higher fertility and rate of immigration, longer life expectancy) these numbers are 74% and 360% (men) and 48% and 209% (women), respectively [17].
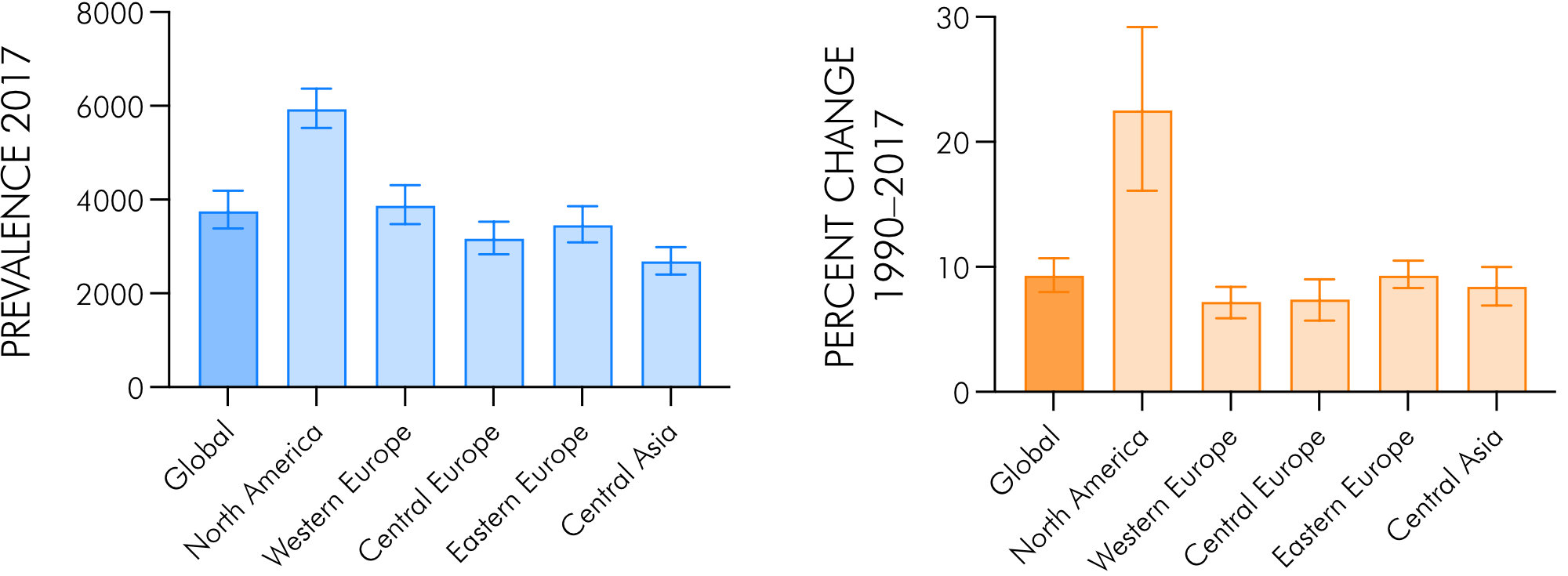
Figure 2. Age-standardized OA prevalence in 2017 per 100,000 population (left panel) and percentage change in age-standardized rates 1990–2017 (right panel), globally and in selected Global Burden of Disease (GBD) regions. Graphs created from data in [9]. Error bars indicate 95% confidence intervals.
Socio-Economic Burden of OA
With its rising prevalence in an ageing and increasingly obese population and the level of impairment, OA is a major personal and socio-economic burden and a significant public health issue. Associated comorbidities such as diabetes and cardiovascular diseases cause additional costs and higher mortality [2, 5, 8, 11]. Age-standardized Years Lived with Disability (YLD) due to OA had been increasing by ~10% between 1990 and 2017 to ~119 years per 100,000 population [9].
According to the Medical Expenditure Panel Survey (MEPS), aggregate annual medical care expenditures in the USA were 185.5 billion USD for the years 1996–2005 including insurer- and out-of-pocket expenditures [18]. For 2012 the Arthritis Foundation estimated OA-related costs of 100 billion USD and earning losses of 80 billion USD per year from 2008–2012 [1]. Annual absenteeism costs to employers were estimated at 10.3 billion USD [19]. Substantial direct and indirect OA-related costs have also been reported from European countries [20, 21]. In Austria, a study in 2008 on 174 subjects (129 female, 45 male) with cox- and gonarthrosis after joint arthroplasty reported overall direct costs of 2, 747 Euros per patient in the year before arthroplasty [22].
Pathogenesis and Diagnosis of OA.
OA is characterized by progressive cartilage damage, inflammation of the synovial compartment (synovitis), subchondral bone alterations such as bone cysts, formation of bone spurs (osteophytes), reduction in joint space width (JSW), i.e., joint space narrowing (JSN) and bone marrow lesions (BMLs). Additionally, the ligaments, fat pads, nerves, tendons, and periarticular muscles can be affected. In face of the whole range of joint tissues being involved, OA is meanwhile understood as a progressive disorder of the whole synovial joint organ eventually leading to “joint failure” [8, 23-27]. In case of knee OA, degeneration of the meniscus plays a central role in the pathological process. Characteristic joint alterations associated with gonarthrosis are depicted in Fig. 3.
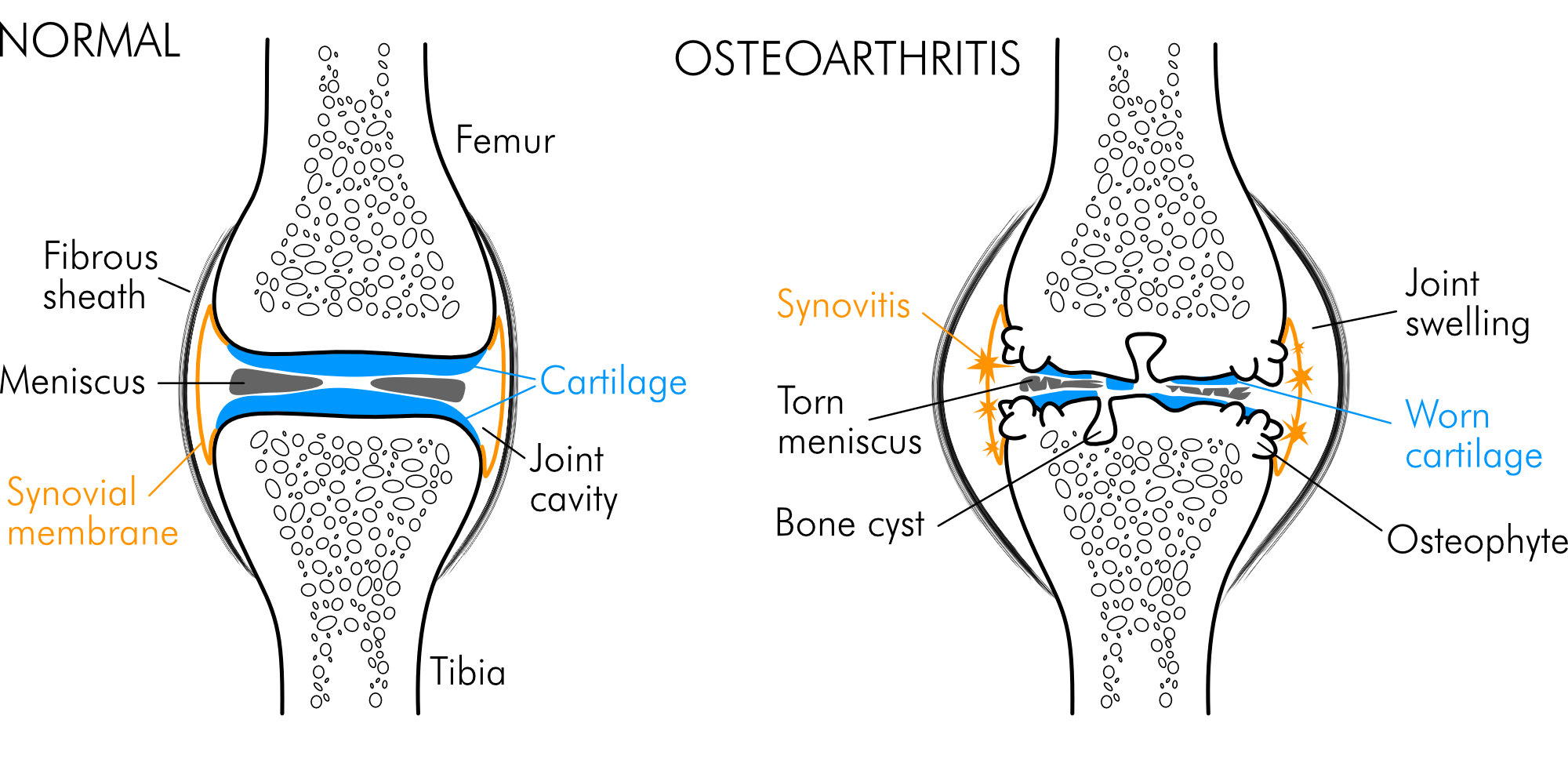
Figure 3. Normal knee and osteoarthritic knee with characteristic structural alterations of joint tissues.
Despite the multifactorial nature of OA and potential differences between the biological mechanisms underlying age-associated, post-traumatic and/or metabolic phenotypes, the pathological changes seen in affected joints have common features [13, 24]. In any case, progressive cartilage degeneration is a central characteristic of OA, with the earliest signs appearing at the joint surface in areas where mechanical forces, especially shear stress, are greatest [24]. Although it is still not clear what triggers the disease, the failure and death of chondrocytes as the only cell type in cartilage is central in the pathogenesis and progression of OA [28].
Under normal conditions, chondrocytes maintain the homeostasis between extracellular matrix synthesis and degradation. Prior to chondrocyte failure, an unsuccessful hypertrophic repair phase occurs in early OA, characterized by increased chondrocyte proliferation, collagen type II and proteoglycan synthesis. This causes cartilage softening, increased tissue hydration and osmotic pressure due to loss of extracellular matrix components (glycosaminoglycans), which sensitizes chondrocytes to impact load and decreases the compressive resistance of the cartilage tissue [6, 26, 29-31]. In a feed-forward mode, the progression of OA is associated with/driven by inflammation (synovitis), release of inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-a (TNF-a), infiltration of lymphocytes and an augmented release of inflammatory mediators. IL-1β may be released upon activation of inflammasomes which were recently unraveled to play a significant role in the development of OA [32]. The release of matrix degenerating proteases including aggrecanases and collagenases – members of the matrix metalloproteinase (MMP) family – causes breakdown of the extracellular matrix [13, 24, 26], which along with pronounced tissue hypoxia, extracellular acidosis and hypoosmolarity induce apoptosis in chondrocytes [33, 34]. Activation of the NLRP3 (NLR family, pyrin domain containing 3; NLR, nucleotide-binding domain, leucine-rich repeat) inflammasome may also induce a distinct form of programmed lytic CD, pyroptosis [35]. Activation of distinct chloride channels and changes of intracellular ion composition appear to play an important role in this process [36]. Moreover, ferroptosis, an iron-dependent mode of cell death characterized by disturbed redox balance and lethal lipid peroxidation, and necroptosis, a caspase-independent programmed form of necrotic cell death, may play a role in the pathogenesis of OA [37].
The reduced production of collagen type II and proteoglycans because of chondrocyte death further destabilizes the cartilage. The amplified inflammatory and catabolic processes cause cartilage remodeling and degradation from superficial fibrillation to deeper clefts (fissures). Damage of adjacent subchondral bone, bone marrow, tendons and muscles and other tissues eventually leads to a loss of joint architecture and joint deformity [8].
Symptoms of OA include pain, stiffness, swelling and loss of joint flexibility. While pain is a leading symptom, radiographic abnormalities may occur in the absence of pain. The prevalence of asymptomatic knee OA in adults aged ≥ 45 years is 11–15% [6, 26, 38]. Diagnosis is mainly based on the symptoms, anamnesis (e.g., family history, history of injury), physical examination, imaging, and, in special cases, laboratory testing [6, 26]. Physical examination comprises palpation, testing on the range of movement and functional tests such as stability of the ligaments, meniscus test and gait analysis. For impairment by pain and loss of joint function, the Western Ontario and McMaster Universities OA Index (WOMAC) questionnaire allows for a reproducible evaluation of the extent of impairment. It is a patient self-administered instrument for evaluation of the status of hip or knee OA, addressing the severity of pain (five questions) and the limitation in physical function (17 questions) as well as the activities of daily life during the past 48 hours and is available either in a psychometric Likert scale version, or in a Visual Analog Scale (VAS) version [39, 40]. The WOMAC has been developed primarily for evaluative research in clinical trials and is not routinely used in the clinical practice despite its validity [39].
X-ray imaging is used for primary diagnosis of OA as well as for monitoring disease progression and has been “traditionally” used for disease staging since 1957 after Kellgren and Lawrence (K–L scale) [41] also in clinical trials. The four-stage K–L scale considers typical signs of OA such as JSN and osteophyte development, with stage 2 as threshold for the presence of OA (Stage 0: no abnormality, stage 1: incipient OA with beginning osteophyte formation, stage 2: moderate JSN and subchondral sclerosis, stage 3: > 50% JSN and extensive subchondral sclerosis and osteophyte formation, stage 4: joint destruction with obliterated joint space, subchondral cysts). However, the K–L system suffers from inexact wording of the descriptors so that there are considerable differences in the classification of OA stages in clinical practice as well as in trials. Schiphof et al. found five different interpretations of the K–L staging system with significant disagreement in major epidemiological studies on knee OA. In some cohort studies, descriptions used in the K–L scale even changed during follow-up [42].
With the broad use of additional imaging techniques beyond X-ray, including magnetic resonance imaging (MRI), ultrasonography and arthroscopy, there has been the call to develop novel criteria for the diagnosis of OA, e.g., by the European League Against Rheumatism (EULAR) [26]. These techniques are superior in visualizing pre-radiographic structural defects of the cartilage, meniscus, ligaments, and synovial membrane [43], which meets the concept of OA of a whole joint disease, and which is paramount for the diagnosis of early stages of OA [26]. Based on better knowledge of the pathophysiology of OA and the associated morphologic alterations, a new system of staging has been elaborated by an Osteoarthritis Research Society International (OARSI) working group. The OARSI Osteoarthritis Cartilage Histopathology Assessment System defines six grades (defining the depth progression into the cartilage; 0: cartilage surface and morphology intact, 1: surface intact, 2: surface discontinuity, 3: vertical fissures, 4: erosion, 5: denudation, 6: deformation), four stages (defining the horizontal extent of the cartilage surface involvement irrespective of the grade; 1: < 10%, 2: 10–25%, 3: 25–50%, 4: > 50%) and a score (an index of combined grade and stage; score = grade × stage) [44]. Despite the validity of this more recent scoring system, the traditional K–L staging is, however, still primarily used for defining inclusion criteria in epidemiologic studies on knee OA.
Treatment of OA.
OA is not curable at present but can be well-managed by treatment. Current therapies are mainly symptomatic and focus on symptom relief [8]. Following the guidelines of the German Society for Orthopedics and Orthopedic Surgery and the German Professional Association of Orthopedists and Trauma Surgeons, the goals of treatment are to mitigate the symptoms, i.e., pain relief, to improve QoL and mobility, and to slow the progression of the disease [6]. Conservative treatment comprises physical and physiotherapeutic measures including spa and low-dose radiotherapy therapy [45, 46], orthopedic aids and orthoses, weight loss if indicated and pharmacotherapy. However, conservative measures are often of limited efficacy due to their onset in already advanced stages of the disease when structural deterioration is often advanced. This underlines the importance of identifying and, if possible, eliminating risk factors, and diagnosis already in early stages of the disease [26]. Joint-preserving surgery is indicated if clinical manifestations persist despite conservative treatment. These measures include arthroscopy (e.g., lavage, shaving), drilling, microfracturing, abrasion arthroplasty and corrective osteotomy near the joint [6]. Joint-preserving measures also include autologous chondrocyte or osteochondral transplantation and – possibly in the future – stem cell-based approaches which are currently being developed [6, 28, 47]. If the possibilities of all these treatments are exhausted, partial- or total joint replacement is the ultima ratio.
Medications used in pharmacotherapy of knee OA are classified as symptomatic rapid-acting drugs of OA (SYRADOAs) such as analgesics and anti-inflammatory drugs, opioids, glucocorticoids, anti-cytokines, or so called slow-acting drugs for OA (SADOAs). If effective, paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) are the first-choice analgesic and anti-inflammatory SYRADOAs [8]. If ineffective or not well tolerated due to adverse, e.g., nephrotoxic side effects, opioids are used instead. Intraarticular (i.a.) injection of corticosteroids is used to treat inflammation and severe pain [6]. SADOA is a comprehensive term coined by the OARSI for substances with delayed and prolonged (carry-over) effects, which can be given orally or are directly applied intraarticularly. SADOA are subdivided into symptomatic slow acting drugs in OA (SYSADOAs) and disease-modifying OA drugs (DMOADs) [6]. Table 1 lists the principal characteristics of the two substance classes.
SYSADOAs are used for symptomatic treatment, but by definition do not have direct analgesic or anti-inflammatory effects such as paracetamol, opioids, NSAIDs or glucocorticosteroids. They can act by inhibiting the synthesis of matrix-degrading proteases, by decreasing the formation of reactive oxygen- and nitrous species, and by protecting the cartilage against pro-inflammatory cytokines [6, 48]. DMOADs can modify disease progression by stabilizing the joint structure, improving physical function, reducing pain and, ideally, by stimulating joint tissue regeneration [27]. Although disease modification is not by definition a hallmark feature of SYSADOAs, the two acronyms are often used synonymously in the literature due to overlapping effects of SYSADOAs and DMOADs. DMOAD-candidates currently under investigation include (i) cartilage matrix precursors, (ii) cytokine modulators, which prevent adverse cytokine effects, e.g., mediated by IL-1β, or TNF-a antibodies, (iii) bisphosphonates, which prevent the decrease in bone density, (iv) matrix metalloproteinase (MMP) inhibitors, and (v) substances stimulating chondrocyte proliferation and cartilage matrix production (collagen type-II and proteoglycan synthesis).
Fig. 4 gives an overview of drugs in clinical use, under development or in evaluation for OA pharmacotherapy. Several potential DMOADs including glucosamine sulfate, chondroitin sulfate, hyaluronic acid, diacerein, risedronate and strontium renalate have been studied in randomized controlled trials (RCTs) for their efficacy in the treatment of knee OA and the results of these trials have been evaluated in systematic reviews and meta-analyses. The results suggest that disease modification by pharmacological intervention might be possible, but the evidence for long-term structural and functional improvement versus placebo controls and/or symptom modification on the WOMAC OA index is weak and inconsistent [8, 40, 49-51]. Despite substantial efforts in drug development programs and clinical trials, no DMOAD has yet been approved by the US Federal Drug Agency (FDA) or European Union regulatory bodies [52].
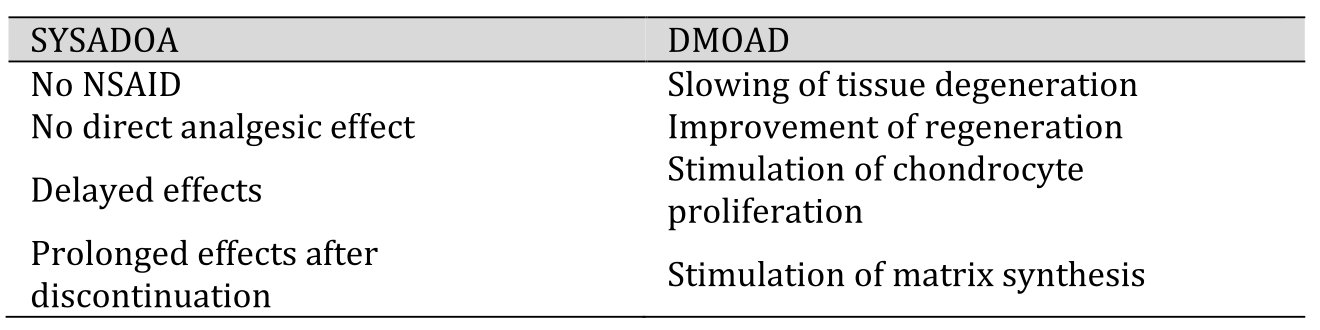
Table 1. Slow-acting drugs for OA (SADOAs). Properties of symptomatic slow acting drugs in OA (SYSADOAs) versus disease-modifying OA drugs (DMOADs) [6, 27]. NSAID (nonsteroidal anti-inflammatory drug)
Due to the high prevalence of OA and the associated substantial OA-related health care costs, personal costs and the impaired QoL for the patients, there is an urgent need for effective DMOADs. To this end, factors like e.g., bone morphogenetic protein-7 (BMP-7), human serum albumin (HSA), interleukin-1 (IL-1) inhibitor, β-nerve growth factor (β-NGF) antibody, matrix extracellular phosphoglycoprotein (MEPE), inverse agonist of retinoic acid-related orphan receptor alpha (RORα) are under investigation and C-type natriuretic peptide (CNP), insulin-like growth factor-1 (IGF-1) and -2 (IGF-2), or the Wingless and Int-1 (Wnt) signaling inhibitor lorecivivint [53] are under consideration [53-55].
Based on encouraging results from preclinical studies and clinical trials in patients, sprifermin has come into focus as a promising DMOAD candidate [56-58]. Sprifermin, also known as recombinant human fibroblast growth factor 18 (rhFGF18), is a truncated 170-amino acid form of FGF18, from which the signal sequence, and the eleven C-terminal amino acids have been removed [59]. Originating from the protein therapeutics developer ZymoGenetics, it was further developed by Merck KGaA/EMD Serono (Merck Serono) under the developmental code name AS-902330 [60] and is the only FGF-based drug currently in clinical trials for targeted treatment in knee OA.
FGFs and FGF Signaling.
Fibroblast growth factors (FGFs) are members of a heterogeneous family of polypeptide mitogens with 15–38 kilodaltons (kDa) in size, classified into six subfamilies (FGF1, 4, 7–9 and 19). All subfamily members act in a paracrine mode, except members of the FGF19 subfamily, which are endocrine factors [61, 62]. FGFs have pleiotropic effects on cell metabolism, development, and tissue homeostasis and are involved in the regulation of cell proliferation, differentiation, survival, and migration (Fig. 5).
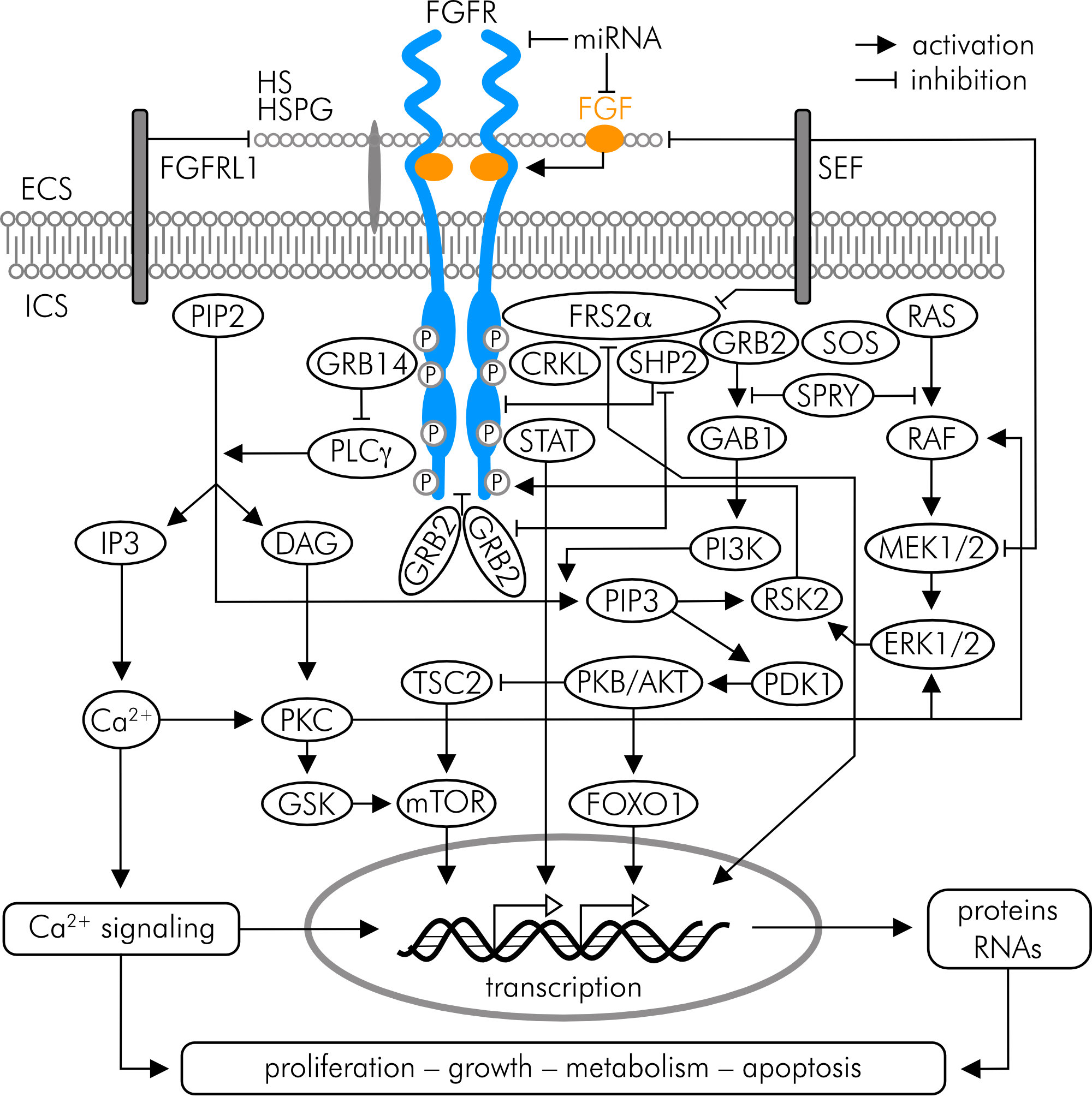
Figure 5. FGF receptor (FGFR) signaling including pathways implicated in bone and cartilage development and homeostasis. ECS (extracellular space), FGFRL1 (FGFR ligand 1), HS (heparan sulfate), HSPG (heparan sulfate proteoglycan), ICS (intracellular space), miRNA (microRNA); SEF (similar expression to FGF). For other abbreviations and details refer to the text and references cited in the text.
FGFs act by binding to FGF receptors (FGFRs transcript variants 1–4), which are members of the receptor tyrosine kinase (RTK) family [61-63]. FGFR1–3 have two splice variants, IIIb and IIIc, which are expressed in epithelial or mesenchymal cells [61]. RTKs are single-pass transmembrane proteins consisting of an extracellular, ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. FGF binding to the inactive, monomeric receptor causes a conformational change, which facilitates receptor dimerization and intracellular tyrosine kinase activation by autophosphorylation. In its dimerized, phosphorylated form, the cytoplasmic tail serves as a docking site for the signaling molecules like phospholipase Cg (PLCg), or FGF receptor substrate 2a (FRS2a), which activate downstream signaling cascades such as the phosphatidylinositol biphosphate (PIP2)/diacylglycerol (DAG)/protein kinase C (PKC), the phosphoinositol-3-kinase (PI3K)/protein kinase B (PKB/AKT), or the Ras/Raf-MEK-MAPK/ERK (mitogen-activated protein kinases/extracellular signal-regulated kinases) pathway [62-64] (Fig. 5).
Role of FGF18/Sprifermin in Bone and Cartilage Development and Homeostasis and the Pathogenesis of OA.
FGF signaling is of fundamental importance for bone and cartilage development and homeostasis by affecting the proliferation and viability of osteogenic-, chondrogenic- and synovial cells. Signaling via FGFR1 and FGFR3 expressed in human chondrocytes exert catabolic and anabolic effects on joint cartilage, respectively [63-65]. Disordered FGF/FGFR signaling contributes to malformations of joints and is strongly implicated in the pathogenesis of OA [62, 65]. A positive correlation was e.g., found between serum FGF1 and FGF2 concentrations and radiographic signs of OA in early disease stages; furthermore, FGF2 and FGF1 expression is increased in the synovial membrane and correlates with OA progression in late stages of the disease [65, 66]. FGF1 exerts its catabolic effects on cartilage by stimulating the expression of MMP13, which causes breakdown of aggrecan, and collagen type II as shown in human articular chondrocytes and cartilage [67].
FGF18, a member of the FGF8 subfamily along with FGF8 and FGF17, and its recombinant form sprifermin (rhFGF18) selectively bind to FGFR3-IIIc in chondrocytes and activate downstream PI3K/AKT and MAPKs to stimulate cell proliferation, survival and function [59, 64, 65, 68, 69] (Fig. 5). This is e.g., evidenced by the finding that cartilage nodule formation is impaired and collagen type II and proteoglycan synthesis in decreased in FGFR3-deficient mice [70]. In 3D culture of porcine chondrocytes permanent sprifermin exposure exerts only transient effects, like ERK1/2 activation. This desensitization is due to internalization of sprifermin via clathrin- and dynamin-independent endocytosis and subsequent lysosomal degradation. However, if cells are intermittently exposed to sprifermin in cycles of one day/week, ERK1/2 activation is reestablished and the effects on cell proliferation and reduction of type I collagen expression are greater compared to permanent exposure [69]. Likewise, in bovine articular chondrocytes the anabolic effect of sprifermin is more pronounced with cyclic exposure [54].
Literature data from clinical trials, in vivo animal studies and in vitro studies were collected, analyzed, and discussed. A systematic literature search was performed in October 2020 and continuously updated until June 2022 in bibliographic databases including PubMed, Google Scholar, Cochrane Central Register of Controlled Trials (CENTRAL) and ClinicalTrials.gov for primary literature, review articles, RCTs, systematic reviews and meta-analyses. Additional sources of primary literature were reports from societies such as the Osteoarthritis Research Society International (OARSI), the European League Against Rheumatism (EULAR), the Arthritis Foundation, or the Osteoarthritis Action Alliance. Literature search was performed using the following search terms, truncations thereof and keyword combinations (Medical Subject Headings [MeSH]) using Boolean operators (AND, OR, NOT): Ageing, articular, arthritis, bone, burden of disease, cartilage, chondrocyte, coxarthrosis, diagnosis, etiology, FGF18, fibroblast growth factor 18, gonarthrosis, joint, matrix, osteoarthritic pain, osteoarthritis, osteoarthrosis, prevalence, prevention, proliferation, sprifermin, staging, subchondral bone, signaling, synovitis, viability, WOMAC
In vitro and in vivo studies have shown beneficial anabolic effects of FGF18/sprifermin on chondrocytes and matrix production, and protective effects against the development and progression of OA in animals [62]. In vitro, the chondrogenic effect of sprifermin was confirmed in human chondrocyte monolayers and 3D cultures, where it stimulated cell proliferation and the number of matrix-producing chondrocytes, respectively, increased the collagen type II/I ratio and stimulated the expression of SOX9, a pivotal transcription factor in cartilage development [59, 71]. These effects appeared to be mediated by FGF18/FGFR3/ERK-signaling (Fig. 5). Reducing FGF18 expression in IL-1β-induced primary human chondrocytes via microRNA (miRNA) interference inhibited cell viability and collagen type II expression, and promoted the secretion of IL-6, IL-8 or TNF-α [72].
Ex vivo, in human and bovine cartilage explants, sprifermin was shown to exert chondrogenic effects by enhancing defect healing and cartilage integrity, by counteracting the loss of depth-dependent mechanical profile of explants, by stimulating collagen type II and proteoglycan synthesis and by suppressing the production of matrix-degenerating MMPs [73-76]. A similar pattern of chondrogenic effects of sprifermin was observed in bovine articular chondrocytes embedded in fibrin-hyaluronan hydrogel and subjected to mechanical loading [77].
In an in vivo rat meniscal tear model of OA, where the meniscus and cartilage are surgically damaged, Moore et al. found a dose-dependent increase in cartilage thickness of the tibial plateau due to new cartilage formation and significantly reduced cartilage degeneration scores in animals receiving i.a. FGF18 [78]. In a rat post-traumatic OA model, i.a. FGF18 attenuates cartilage degeneration, stimulates collagen type II synthesis, and suppresses MMP13 [68]. On the cellular level, this study found that FGF18 promoted chondrocyte proliferation and migration, and counteracted IL-1β-induced chondrocyte apoptosis, disruption of the mitochondrial membrane potential and mitochondrial morphology via PI3K-AKT signaling. Furthermore, the FGFR1/FGFR3 ratio was upregulated by IL-1β and downregulated by FGF18 [68]. In line with these findings, Tang et al. previously found in knee joints of adult mice, that knockout of FGFR3 led to an upregulation of MMP13 and downregulation of collagen type II, whereas FGFR3 activation attenuated cartilage degradation [79]. In pharmacokinetic studies in rats, it could be shown that sprifermin was preferentially concentrated in the target joint after i.a. injection and was still detectable up to 28 days post-injection, while its levels in other organs and tissues were low and no intact, undegraded sprifermin could be detected in the systemic blood [80]. Recently it was shown in a surgical mouse OA model by 99mTc-NTP 15-5 SPECT-CT imaging, which allows for monitoring of proteoglycan remodeling, that under sprifermin 99mTc-NTP 15-5 uptake in OA knees was significantly increased compared to controls in parallel with proteoglycan [81]. In fetlock joints of horses, in which a cartilage defect was induced, and which were thereafter subjected to microfracture treatment (a common surgical technique to enhance cartilage healing at the site of injury), i.a. application of sprifermin has been shown to enhance the effects of this form of treatment, suggesting a cartilage-regenerative role of sprifermin treatment in early cartilage injuries [82]. In human bone marrow mesenchymal stem cells (hBMSCs) sprifermin promotes chondrogenic differentiation in vitro and in a rat model of rotator cuff it improves tendon-to-bone healing [83], and in an in vivo rabbit annular model of intervertebral disc (IVD) degeneration this process was inhibited by lentivirus-mediated transfer of gene encoding fibroblast growth factor which caused suppression of apoptosis of nucleus pulposus cells as well as expression of matrix‑degrading enzymes [84].
Taken together, there is strong evidence coming from in vitro studies and animal experiments for the beneficial anabolic effects of exogenous FGF18/sprifermin on chondrocyte viability and function, matrix turnover, cartilage mass and integrity. The data also highlight the importance of the FGF18/FGFR3 axis in targeting cartilage regeneration and repair in OA.
Sprifermin is currently the only potential FGF-based DMOAD that has been used in clinical trials for knee OA [56-58]. Five RCTs have yet been initiated – sponsored by Merck KGaA/EMD Serono (Darmstadt, Germany), two were terminated due to low recruitment. Three RCTs have been completed (Table 2). All published post hoc analyses and follow-up data are based on these three original trials.
Here we address the following questions based on available RCT data: (i) Is there evidence for the efficacy of sprifermin in the treatment of symptomatic knee OA regarding structural improvements on cartilage and non-cartilage joint tissues? (ii) Is sprifermin administration associated with specific adverse effects? (iii) Do the available trial data allow to infer that sprifermin treatment in knee OA can improve the outcomes of pain, articular stiffness, and physical joint function? (iv) What are the limitations of the RCTs with respect to the sizes of the study populations, inclusion and exclusion criteria, controls, endpoints, and biomarkers? The review traces the path from the phase 1 First-in-Human (FiH) study initiated in 2007 (ClinicalTrials.gov NCT00911469) over a phase 1 placebo-controlled RCT (NCT01033994) to the so far highest developmental phase 2b FGF18 Osteoarthritis Randomized Trial with Administration of Repeated Doses (FORWARD) study (NCT01919164) completed in 2019 (Table 2).
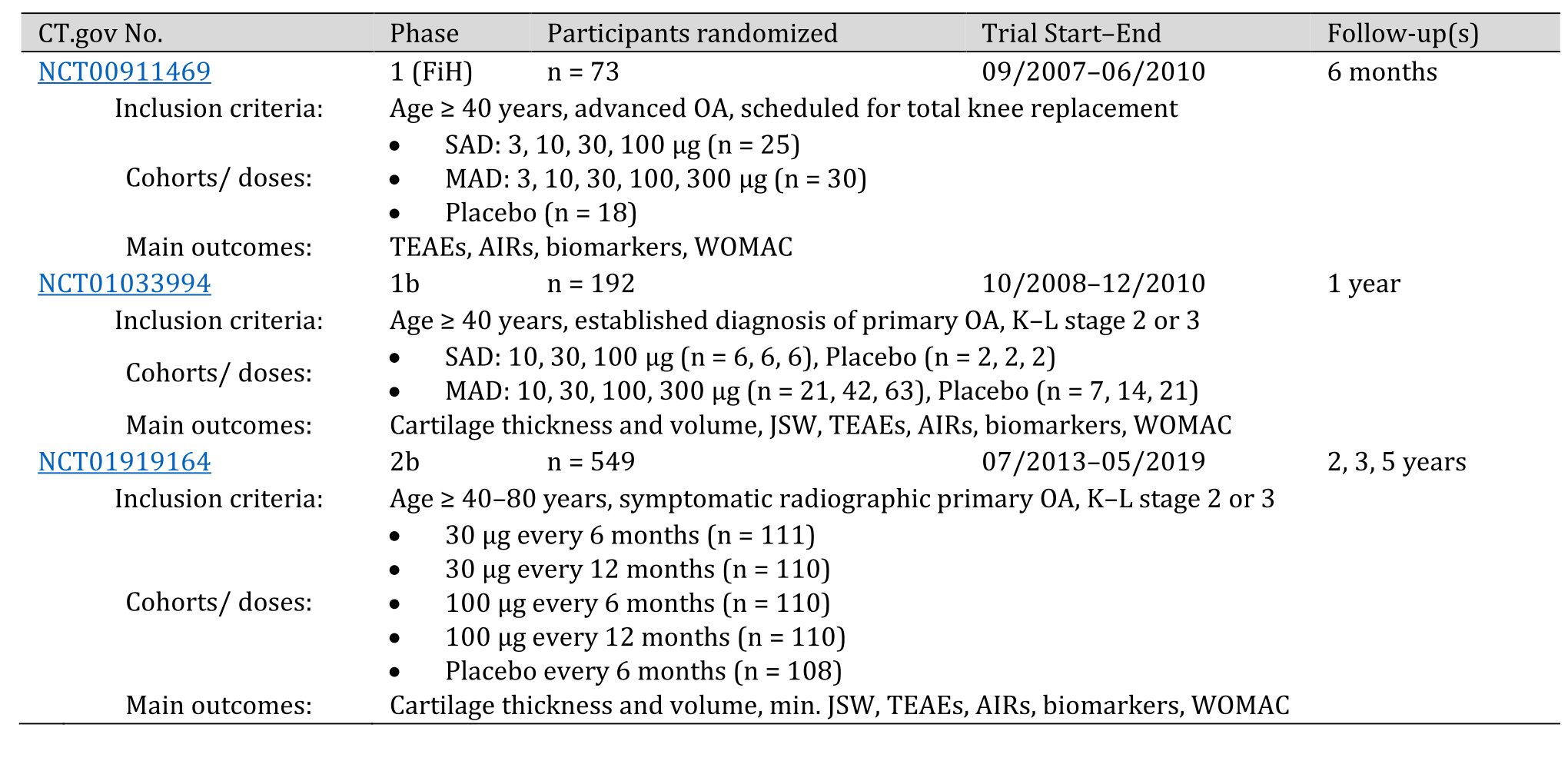
Table 2. Randomized placebo-controlled trials on sprifermin (rhFGF18) in knee OA. AIRs (acute inflammatory reactions); CT.gov (ClinicalTrials.gov); JSW (joint space width); K–L (Kellgren–Lawrence stage); MAD (multiple ascending dose); SAD (single ascending dose); TEAEs (treatment-emergent adverse events); n = numbers of patients at randomization (baseline)
Phase 1 Trials
The phase 1, FiH trial on sprifermin entitled “Study of AS902330 (rhFGF-18) Administered Intraarticularly in Patients with Knee Primary Osteoarthritis Who Are Candidates for Total Knee Replacement” (NCT00911469) was a multicenter randomized, double-blind, placebo-controlled trial performed in patients ≥ 40 years with advanced primary femorotibial knee OA for at least six months scheduled for total knee replacement. The aim was to evaluate the safety of single and multiple ascending doses of i.a. sprifermin (primary outcome). Randomization was 3:1 for sprifermin or placebo, and sprifermin doses were either 3, 10, 30, 100 or 300 µg i.a. injection per subject in the single ascending dose (SAD) cohorts and 10, 30, 100, 300 µg injection per week for three weeks per subject in the multiple ascending dose (MAD) cohort. In total, 73 participants (n = 73) were recruited for this study, 55 received sprifermin (25 with SAD, 30 with MAD) and 18 received placebo (Table 2). The follow-up was six months. Primary outcome measures were the nature, incidence, and severity of treatment-emergent adverse events (TEAEs), the proportion of patients with local adverse events (AEs) such as acute inflammatory reactions (AIRs) associated with pain and self-reported synovial fluid effusion, local tolerability in the target knee, laboratory safety parameters (blood chemistry, hematology, urinalysis) and electrocardiography (ECG). Secondary outcome measures included biomarkers of cartilage anabolism and catabolism, changes in levels of cytokines related to inflammation (IL-1β, IL-6, IL-8, and TNF-a), blood levels of sprifermin and the presence of anti-sprifermin antibodies. The results showed no difference between the experimental and placebo group in TEAEs, local AEs, local tolerability, pain, swelling in the knee, laboratory parameters or ECG (AIR were slightly more common with 300 µg sprifermin). Pharmacokinetic analyses showed no sprifermin or anti-sprifermin-antibodies in the systemic blood after i.a. injections. Overall, this FiH study raised no serious safety concerns about i.a. sprifermin application in OA patients [85].
A second phase 1, randomized, double-blind, placebo-controlled trial on the efficacy and safety of i.a. sprifermin entitled “A Multicenter Study of rhFGF 18 in Patients With Knee Osteoarthritis Not Requiring Surgery” (NCT01033994) was conducted at 30 sites on three continents on patients aged ≥ 40 years with a diagnosis (≥ six months) of primary femorotibial knee OA (K–L stage 2 or 3) according to the American College of Rheumatology clinical and radiologic criteria. Eligible patients were randomized 3:1 for sprifermin or placebo. Sprifermin was injected i.a. into one or both knees at either 10, 30 or 100 µg per subject in the SAD cohort and either 10, 30 or 100 µg per patient per week for three weeks in the MAD cohort (Table 2). MRI images were acquired after randomization (baseline) and three, six, and 12 months after treatment. The primary efficacy outcome measure was the change in central medial femorotibial compartment cartilage thickness at six months and 12 months determined by quantitative MRI (qMRI). The primary safety endpoints were nature, incidence, and severity of local and systemic TEAEs, AIRs, laboratory safety parameters (blood chemistry, hematology, urinalysis) and ECG. Secondary outcome measures included change in WOMAC function and pain index scores, change in OA pain on a VAS scale, change in joint space width based on X-ray, as well as blood levels of sprifermin and the presence of anti-sprifermin antibodies. Of the 192 patients randomized, 180 completed the study, and 168 were analyzed for the primary efficacy outcome on central medial femorotibial compartment cartilage thickness (n = 168), which at 12 months was not significantly different between sprifermin and placebo [86]. However, sprifermin was associated with a statistically significant, dose-related reduction in the loss of total and lateral femorotibial cartilage thickness and volume and in JSW narrowing in the lateral femorotibial compartment in the target knees. So, although the primary efficacy endpoint missed significance, prespecified structural secondary endpoints showed significant dose-dependent beneficial long-term effects of i.a. sprifermin treatment on cartilage thickness. WOMAC pain scores improved in all groups of patients including placebo-injected subjects, with significantly less improvement at 12 months in patients injected with 100 µg sprifermin as compared to placebo. As for safety endpoints, there was no significant difference in TEAEs, serious AEs, or AIRs between experimental and control groups. No anti-sprifermin antibodies were detected in the patients’ systemic blood [86].
For these promising results on structural improvement in knee OA after sprifermin treatment, post hoc analyses on additional structure endpoints have been performed based on imaging data of this trial. Eckstein et al. used subject-specific, location-independent qMRI analysis of cartilage change in 16 joint subregions, to test if sprifermin increases cartilage thickness, and reduces cartilage thinning in target knee, irrespective of the joint region analyzed [87]. Baseline characteristics and subregional cartilage thickness after 12 months follow-up among patients treated with 100 µg sprifermin (n = 57) and matched placebo-treated patients (n = 18) were analyzed. The results of this study show a statistically significant decrease of the total cartilage thinning score, and an increase in the total cartilage thickening score, confirming the anabolic effect of i.a. sprifermin as previously published [87]. A second post hoc analysis on structural outcomes of this trial was performed by Roemer et al., in which the effect of sprifermin on various joint tissues was assessed by semi-quantitative WORMS scorings from MRI [88]. Besides cartilage morphology, this study also assessed the effect of sprifermin on other joint tissues such as BMLs, menisci, osteophytes, and synovitis. The analyses focused on changes in the 100-µg subgroup of patients (n = 57) and matching placebo-injected patients (n = 18), as in the previous post hoc analysis by Eckstein et al [87].. Significantly less cartilage degradation was observed from baseline to 12 months in the patello-femoral joint compartment versus placebo. BMLs showed significantly more improvement from six to 12 months for the whole knee versus placebo, but not from baseline. Other structural outcomes such as menisci, osteophytes, and synovitis showed no significant changes [88].
Phase 2 Trials
Two phase 2 trials on sprifermin in knee OA were initiated in 2010 and 2013, respectively, but both were terminated due to low recruitment. Nonetheless these studies are mentioned here to give a complete overview on the trials that have been yet initiated for further clinical validation of the drug. The “Sprifermin (AS902330) in Cartilage Injury Repair (CIR)” trial (NCT01066871) with the official title “A Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group Phase II Trial to Investigate the Efficacy and Safety of Weekly Intra-articular (i.a.) Injections of 10, 30, and 100 µg of AS902330 for Three Consecutive Weeks in Patients With Acute Cartilage Injury of the Knee” (03/2010–04/2013) had the percent change from baseline in cartilage defect volume at month 12 defined as primary outcome, and several efficacy and safety measured defined as secondary endpoints. This trial has data available of the 74 recruited participants at ClinicalTrials.gov, which, however, have not been published elsewhere. The RCT entitled “A Multicenter Trial of Sprifermin (AS902330 [Recombinant Human Fibroblast Growth Factor-18]) or Placebo After Microfracture Surgery for Cartilage Injury of the Knee” (NCT01689337) (04/2013–10/2013) had the evaluation of the effect of i.a. sprifermin on the composition of the refilled cartilage in the target knee after microfracture surgery, and the assessment of the safety profile of sprifermin injection defined as primary objectives, but was terminated as only one participant could be recruited.
The FORWARD study (“A Study to Investigate the Safety and Effectiveness of Different Doses of Sprifermin in Participants With Osteoarthritis of the Knee (FORWARD)”; NCT01919164) initiated in 2013 is the only completed phase 2b trial on the efficacy and safety of i.a. sprifermin in knee OA. It was a dose-finding, multicenter (12 sites in the European Union, USA, and Asia), double-blind, placebo-controlled, parallel-group RCT in participants aged ≥ 40–85 years, with primary knee OA and K–L stage 2 or 3. The 549 enrolled intention-to-treat (ITT) participants (69% female) were equally randomized to each of the four treatment arms, or a placebo arm. The treatment phase starting at randomization (week 0) lasted for two years and had a three-year extended follow-up phase. The participants received 30 µg i.a. sprifermin every six or 12 months (n = 111 and 110, respectively), 100 µg i.a. sprifermin every six or 12 months (n = 110 each), or placebo every six months (n = 108) (Table 2). Each treatment consisted of an injection once a week over three weeks. The primary endpoint was the change from baseline in cartilage thickness in the total femorotibial joint as evaluated by qMRI at year two. The main secondary endpoints included the two-year change from baseline in the total WOMAC score as well as on pain, stiffness, and physical function subscales, change from baseline in minimal joint space with assessed by X-ray, change from baseline in cartilage volume and thickness in medial and lateral compartments, TEAEs, AIRs, synovial fluid and serum levels of sprifermin and development of anti-sprifermin antibodies. Endpoints analyzed after three years were not prespecified and were considered exploratory. These endpoints were change from baseline in total, medial, lateral, central medial, and central lateral femorotibial joint cartilage thickness measured by qMRI, medial and lateral minimum joint space width assessed by X-ray, and WOMAC total score. The results of the trial were published in JAMA in 2019 [52]. Primary data had been published earlier in abstract form [89, 90]. The results for the primary endpoint showed a dose-dependent improvement of total femorotibial joint cartilage thickness after two years versus placebo, which was statistically significant in the 100-µg sprifermin cohorts receiving sprifermin every six or every 12 months. The results for the secondary structural endpoints showed significant dose-dependent increases in cartilage thickness and volume in the medial and lateral compartment over two years. Joint space width was significantly affected only in the lateral compartment versus placebo and among the 30-µg and 100-µg sprifermin cohorts. Regarding symptomatic secondary endpoints, there were no statistically significant differences in change from baseline neither in total WOMAC scores, nor in pain, function, or stiffness subscale scores for any sprifermin group versus placebo. TEAEs were reported by over 90% of participants but were mostly mild and unrelated to treatment with sprifermin or placebo. AIRs were reported in ~14–23% (higher in the 100-µg sprifermin groups), but none led to discontinuation. In total, 38 patients tested positive for anti-sprifermin antibodies, six of which were in the placebo group. The three-year explanatory follow-up evaluation of efficacy on 442 participants showed an overall decrease in total femorotibial cartilage thickness between years two and three. The difference in cartilage thickness from baseline after three years remained significantly different between placebo and the group that had received 100 µg sprifermin every six months. There were no significant differences in the mean absolute change from baseline in total WOMAC scores in any sprifermin group versus placebo [52].
In a post hoc analysis of qMRI data of all 549 participants of the FORWARD study, Roemer et al. reported a dose-dependent effect on semi-quantitatively scored cartilage morphology changes over two years with sprifermin across the entire knee, while analyses for individual compartments did not reach statistical significance [91]. The authors extended their analysis on further joint structures and OA-related parameters for worsening, including BMLs, osteophytes, menisci, and synovitis. BMLs showed a dose-dependent improvement from baseline with higher doses of sprifermin (100 µg every six or 12 months) versus placebo after two years, while no differences between sprifermin and placebo were observed for osteophytes, meniscus damage or synovitis. The results on structural cartilage modification could also be confirmed in a further post hoc study on imaging data of the total FORWARD trial population (n = 549) by employing an automated, machine learning method for cartilage segmentation [92]. This study analyzed cartilage thickness at baseline and at least one post-treatment MRI acquired after six, 12, 18 and 24 months. Using this automated method, the authors could confirm statistically significant, dose-dependent structural improvements under sprifermin over placebo for total femorotibial joint cartilage thickness and for lateral and medial femorotibial subregions. For the 100-µg dose they could also show significant structural improvement in the central medial tibial, central lateral tibial, and central lateral femoral regions [92]. By calculating cartilage thinning/thickening scores for subregional cartilage thickness, Eckstein et al. further expanded the FORWARD trial results. This study showed that over two years patients treated with 100 µM sprifermin every six months lost significantly less and gained more cartilage thickness in all 16 analyzed joint subregions versus placebo-injected patients; the greatest differences were found in the central lateral femoral and central medial tibial compartments [93]. The indirect comparison with thinning and thickening scores from healthy reference subjects from the Osteoarthritis Initiative indicated that cartilage thinning in FORWARD trial patients receiving 100 µM sprifermin was almost reduced to the level of healthy subjects in the same time frame [93].
These post hoc studies on data of the FORWARD trial were focused on structural outcomes with respect to total femorotibial cartilage thickness or joint cartilage subregions and could show clear dose- and time-dependent effects of sprifermin versus placebo. In the most recent analysis, symptomatic outcomes (WOMAC pain scores) have been analyzed in addition to cartilage thickness in a “subgroup at risk” in the FORWARD trial population [94]. The subgroup, consisting of 161 (29%) of all 549 ITT patients, was defined by baseline medial or lateral minimum joint-space width of 1.5–3.5 mm and a WOMAC pain score of 40–90 (moderate-to-high pain). Data up to three years of follow-up were included in the analyses. Structural analysis showed that the net loss in total femorotibial joint cartilage thickness over two years was higher in the “subgroup at risk” as compared to ITT patients, while net cartilage thickness gain in patients receiving 100 µg sprifermin every six months was similar in the “subgroup at risk” and in ITT patients. In the ITT population, WOMAC pain scores after three years were not significantly different between patients treated with sprifermin (100 µg every six months) and placebo, consistent with the original report [52]. However, as a major result of this subgroup analysis, a dose-dependent improvement in WOMAC pain was observed in the “subgroup at risk” treated with sprifermin versus placebo compared to the whole ITT population [94]. The authors concluded that this more homogenous subgroup of patients with moderate-to-high pain at baseline and more rapid progression of cartilage degeneration might potentially benefit more from sprifermin treatment not only with respect to structural (cartilage thickness), but also symptomatic (pain reduction) disease modification.
Eckstein et al. reported the five-year efficacy and safety results of the FORWARD trial [95]. Of the 549 ITT participants, 378 (69%) completed the follow-up. The results show that significantly higher total femorotibial joint cartilage thickness was maintained over five years in the cohort that had received 100 µg sprifermin every six months versus placebo and no patient in this cohort had replacement of the target knee within the follow-up period. Mild or moderate AEs were reported by 96–98% and 98% of patients receiving sprifermin or placebo, respectively, but were considered unrelated to treatment. Less than 10% of AEs led to discontinuation. Like in previous follow-ups, there was no difference in WOMAC pain scores between sprifermin- and placebo-treated patients in the ITT population (pain scores improved by ~50% from baseline in all groups). However, the beneficial difference in the WOMAC pain score between sprifermin (100 µg every six months) and placebo was maintained to year five in the previously defined “subgroup at risk” with 161 patients [94].
Bay-Jensen and colleagues recently measured PRO-C2, a serum marker of type II collagen formation (with low levels being prognostic of radiographic progression) in the synovial fluid from participants of the FORWARD study and analyzed if the response in subjects with high or low PRO-C2 levels respond differently to sprifermin. It was shown that PRO-C2 increased over time in response to sprifermin, but not to placebo, and that patients with low serum PRO-C2 levels lost more cartilage thickness over time and grew more cartilage in response to sprifermin versus placebo than patients with high PRO-C2 levels [96].
In view of the individual and societal burden of disease, the unmet need for an efficient, disease-modifying remedy in the treatment of OA is a serious problem for patients, health systems and for the society as a whole [27]. Massive efforts have been put forward in the development and testing of DMOADs, but there are no approved drugs available to date for use in the clinical practice. The DMOAD candidate sprifermin has been tested in three RCTs (Table 2) for its efficacy and safety for the targeted treatment in knee OA and data available from these trials provide strong evidence that sprifermin has significant beneficial effects on cartilage thickness and volume in knee OA patients [52, 58, 86-88, 92, 93, 95]. RCT data are in line with studies in vitro and in animals that have shown beneficial anabolic effects of sprifermin on chondrocytes and matrix production, and protective effects against the development and progression of OA in animals [59, 62, 68, 71, 73-78]. The durability of the structural improvement of cartilage to sprifermin remains uncertain. However, results of the five-year follow-up of the FORWARD trial show the maintenance of morphological improvements up to year five [95], which suggests sustainability of sprifermin treatment for several years post-treatment. Extended post hoc analyses and a meta-analysis of RCT data also suggest a time- and location-dependent improvement at higher sprifermin doses of subchondral BMLs, but the results are inconsistent, and no ameliorating effects of sprifermin versus placebo were found for other joint structures affected in OA such as osteophytes, menisci or the synovial membrane [58, 88, 91].
Regarding safety outcomes, RCT data provide strong evidence that i.a. injections of sprifermin do not exert any specific local or systemic adverse effects compared to placebo-injected cohorts [52, 58, 85, 86, 95]. In contrast to the evidence for anabolic effects of sprifermin on knee joint cartilage and its efficacy to improve cartilage morphology in knee OA patients, there is no evidence that sprifermin causes significant improvements in terms of clinical symptoms or physical function over placebo in whole study populations [52, 58, 86]. WOMAC index scores showed pain reduction in both sprifermin- and placebo-injected cohorts. Pain reduction also in placebo-injected patients has frequently been observed in studies on i.a. DMOAD candidates and the development of strategies to reduce placebo responses will be a major challenge in designing future clinical trials [8, 27, 40, 97]. So, while there is definitely potential in terms of the structural efficacy of sprifermin, the clinical importance of statistically significant morphological improvements and slowing of cartilage degradation remains uncertain, if there is no significant symptomatic benefit [8, 52]. The inefficacy to alleviate pain is probably due to the lack of improvement by sprifermin in other joint tissues than cartilage such as subchondral bone, menisci, and the synovium (and its inflammation), which are more important for symptom generation in OA [8].
From the available clinical data summarized above and the before-mentioned specific requirements for a sound evaluation of the full clinical and socio-economic potential of sprifermin as a DMOAD, improvements for designing and conducting future RCTs can be inferred like larger cohorts of OA patients, longer follow-up periods, use of state-of-the-art diagnostic (imaging) methods and use of improved scoring/grading systems of OA disease states. The heterogeneity of OA is a major challenge to developing DMOADs. It seems unlikely, that one single drug can be effective in the treatment of all subtypes of OA from primary “wear and tear” OA to the diverse forms of secondary OA. All data currently available on safety and efficacy of i.a. sprifermin in knee OA originate from three original RCTs on 814 patients in total (NCT00911469; NCT01033994; NCT01919164) (Table 2). RCTs with larger patient cohorts and longer follow-ups are needed to account for the heterogeneity of the disease as they would allow for subgroup analysis of e.g., age-, sex- or risk-factor-specific outcomes, or stratification of patients during randomization by considering confounding variables. This would possibly allow to identify differences in the response characteristics to sprifermin between different subgroups and meta-analyses of RCT outcomes might reveal disease patterns for prognoses on the subgroup-specific benefits of sprifermin treatment. Indeed, post hoc analysis of a more homogenous “subgroup at risk” in the FORWARD trial population with more severe morphological and symptomatic manifestations could show, that these patients benefited more from sprifermin treatment over placebo in terms of structural improvement and symptomatic alleviation compared to the overall study population [94]. This benefit was maintained over five years post-treatment, suggesting a possible translation of structural improvement to clinical benefit [95].
X-ray is still considered the current “gold standard” for morphological diagnosis of knee OA and so far, only patients with radiographically evident stages of OA (K–L stage 2 or 3) have been included in RCTs. The methodological advances through MRI and ultrasound, allowing for more reliable diagnosis and scoring, and the identification of new and more sensitive biomarkers may help to redesign future RCTs and to identify DMOAD-responder subtypes depending on the etiology of the disease [8, 26, 27, 97]. These more valid methods may not only overcome the limitations and inconsistencies of the K–L staging system [42], but particularly allow to define new criteria for the diagnosis of early knee OA and detection of pre-radiographic cartilage, bone marrow and meniscus lesions [26]. Early diagnosis is considered as paramount for identifying patients at high risk of OA progression, for the initiation of early proactive treatment interventions and the development of treatment regimen tailored to the patients’ individual disease courses [8, 26]. Possibly, in early OA stages patients could benefit more from DMOAD treatment to slow down disease progression and to delay the onset of symptomatic OA and hence to mitigate the overall burden of disease. Testing such treatments (e.g., i.a. sprifermin) in early-stage OA in RCTs probably requires a refinement of study endpoints and more sensitive measures than the current structural and symptomatic outcomes [8].
Despite the encouraging results from RCTs, it would be inappropriate to put exaggerated hope into sprifermin or any other DMOAD candidate as a “miracle drug” which can halt or even reverse disease progression. Disease-modifying OA drugs should rather be seen as part in a bundle of measures along with earliest possible OA diagnosis and – first and foremost – primary prevention, which is still in its infancy despite the increasing burden the disease poses on the society and health care systems [8]. The awareness of the population and policy makers for risk factors triggering the disease such as obesity, joint misalignment, lifestyle habits and injuries must be improved, and all efforts need to be made to fulfill first-line evidence-based public health interventions for OA. These include weight management strategies, physical activity, injury prevention and self-management education [6, 8, 10, 12, 23, 98]. Even if these strategies may not prevent the onset of symptomatic OA, public health programs addressing modifiable risk factors and programs tailored to age, sex and personal coping capabilities could help to preserve or significantly improve the patients’ QoL and reduce the socio-economic burden of the disease [10, 98]. In a bigger picture, along with disease prevention programs, early diagnosis, extended and improved future clinical trials (as outlined below), and therapeutic measures from conservative treatment and pharmacotherapy to surgery, sprifermin, or other DMOAD candidates could become an integral part in OA management in helping to slow down disease progression (Fig. 6).
Figure 6. Integrative approach for management and treatment of OA. Orange color highlights sprifermin as a potential pharmacologic part of an integrative approach to manage OA. Own illustration.
The available results from preclinical studies and clinical trials are encouraging and provide convincing evidence that i.a. injection of sprifermin/rhFGF18 has a sustained anabolic effect on the cartilage in the target knee and causes structural improvements in patients with knee OA, while having no specific local or systemic adverse effects. There is evidence, that the structural improvements can translate into clinical benefit in terms of pain alleviation in knees with severe OA. There is justified hope that an effective disease-modifying therapy might soon be available for the increasing number of patients suffering from OA, and sprifermin can be regarded as a promising DMOAD candidate in knee OA. Still there are significant challenges along the way towards its regulatory approval. Larger RCTs with longer follow-up periods and more sensitive outcome measures and biomarkers are needed to prove its efficacy. More consistent study designs and survey methodologies are required for meaningful cost-effectiveness calculations to estimate the socio-economic benefit of its therapeutic use in terms of reduced health care expenditures and improved QoL. Importantly, instead of putting hope in sprifermin as “magic bullet” in the combat against OA, it should be seen as part of a wide range of measures including disease prevention programs, early diagnosis, conservative treatment and other pharmacotherapeutic approaches.
AE, Adverse Event; AIR, Acute Inflammatory Reaction; BML, Bone Marrow Lesion; BMSC, bone marrow mesenchymal stem cell; CDC, Centers for Disease Control and Prevention; CENTRAL, Central Register of Controlled Trials; COX, Cyclooxygenase; DMOAD, Disease-Modifying Osteoarthritis (OA) Drug; EULAR, European League Against Rheumatism; FDA, Federal Drug Agency; FGF18, Fibroblast Growth Factor 18; FGFR, Fibroblast Growth Factor Receptor; FiH, First-in-Human; GDP, Gross Domestic Product; i.a., intraarticular; ITT, Intention-To-Treat; IVD, Intervertebral Disc; JSN, Joint Space Narrowing; JSW, Joint Space Width; MAD, Multiple Ascending Dose; MeSH, Medical Subject Headings; NSAID, Nonsteroidal Anti-Inflammatory Drug; OA, Osteoarthritis; OARSI, Osteoarthritis Research Society International; qMRI, Quantitative Magnetic Resonance Imaging; QUALY, Quality-adjusted life year; QoL, Quality of Life; RCT, Randomized Controlled Trial; rhFGF18, recombinant human Fibroblast Growth Factor 18; SAD, Single Ascending Dose; SADOA, Slow-Acting Drug for Osteoarthritis (OA); SYRADOA, Symptomatic Rapid Acting Drug of Osteoarthritis (OA); SYSADOA, Symptomatic Slow Acting Drug in Osteoarthritis (OA); TEAE, Treatment-Emergent Adverse Event; VAS, Visual Analog Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis (OA) Index; YLD, Years Lived with Disability.
The authors a grateful to Felix Eckstein5,6,7 for continuous support and helpful discussion.
Author Contributions
MJ was responsible for all aspects of the project, including design and implementation of the research, data analysis, manuscript preparation, and primary responsibility for the final content. AvdZ, WW and MR provided essential expertise in data interpretation and supported manuscript preparation. Supervision, MJ; Validation, AvdZ, MR, WW and MJ; Visualization, MJ; Writing and editing, AvdZ, WW, MR and MJ. All authors have read and approved the final manuscript.
Funding Sources
None.
Statements
The manuscript of this study is based on the academic expert thesis of the first author as part of his university course “Health Sciences and Leadership” at the Paracelsus Medical University (submitted in August 2020).
The authors have no conflicts to disclose.