

Original Article - DOI:10.33594/000000629
Accepted 17 March, 2023 - Published online 17 May, 2023
1Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia;
†Deceased
Background/Aims: Cultured skeletal muscle cells subjected to electrical pulse stimulation (EPS) are widely employed as an in vitro model of exercising skeletal muscle. Numerous studies demonstrated that sustained excitation of skeletal muscle results in the dissipation of the transmembrane gradient of monovalent cations. During exercises the impending loss of excitability has to be counterbalanced by rapid restoration of the Na+i/K+i ratio. To understand mechanisms of the maintenance of muscle contractility, it is necessary to know which transporters are participated in the dissipation of Na+i and K+i gradients and how to activate Na,K-ATPase for its regeneration. This study was aimed at the identification of ion transporters involved in the dissipation of the transmembrane gradients of Na+ and K+induced by EPS, and Na,K-ATPase isoforms involved in its restoration. Methods: The differentiated C2C12 myotubes were subjected to electrical pulse stimulation in the presence or absence of different ion transport systems inhibitors followed by measurement of intracellular monovalent cations by flame atomic absorption spectrometry. Results: Electrical pulse stimulation of C2C12 myotubes results in the dissipation of Na+i/K+i gradient, which is maintained by α2-Na,K-ATPase. Na-K-2Cl cotransporter (NKCC), voltage-gated Na+ (Nav), and large conductance Ca2+-activated K+ channels (BKCa), and Na/H exchanger (NHE) are involved in the dissipation of this gradient. Suppression of calmodulin-dependent protein kinase II (CaMKII) increases Na+ efflux in EPS-treated C2C12 myotubes. Conclusion: NKCC, Nav, BKCa, and NHE are involved in the dissipation of Na+i/K+i gradient in EPS-treated C2C12 myotubes.
Electrical pulse stimulation (EPS) of murine differentiated myotubes (C2C12 cells) results in the dissipation of transmembrane gradient of monovalent cations and elevation of the Na+i/K+i ratio. It resulted in the change of the genes transcription by Ca2+-mediated and Ca2+-independent manner. Inhibitor analysis was used to reveal a relative impact of α1- and α2-containing isoforms of Na,K-ATPase in the maintenance of Na+i/K+i gradient in EPS-treated myotubes, and ion transporters involved in their dissipation. Data obtained in this study demonstrate that: 1) EPS increased the relative impact of α2-Na,K-АТPase in the maintenance of transmembrane Na+i/K+i gradientsin C2C12 myotubes; 2) EPS increased activity of Na-K-2Cl cotransporters measured as ouabain-resistant, bumetanide-sensitive component of Rb+ influx; 3) dissipation of transmembrane Na+i/K+i gradients observed in EPS-treated C2C12 myotubes is mainly due to the activation of tetrodotoxin-sensitive voltage-gated Na+ channels (Nav), Na/H exchanger (NHE) and charibdotoxin-sensitive large conductance Ca2+-activated K+ channels (BKCa), respectively. Inhibitor of voltage-gated Kv1.2 and Kv1.3 K+ channels noxiustoxin and inhibitor of ATP-sensitive K+ channels glibenclamide as well as the inhibitors of intermediate and small conductance Ca2+-activated K+ channels (TRAM-34 and apamin, respectively) did not affect the loss of K+i induced by EPS. Thus, we can suggest that these channels do not participate in the dissipation of K+i gradient induced by EPS.
We also used inhibitor analysis to identify elements of the EPS-activated signaling system(s). The data obtained indicate that calmodulin-dependent protein kinase II suppresses Na+ efflux through Na,K-ATPase and/or other ion-transport system(s). Nicardipine (an inhibitor of L-type voltage-gated Ca2+ channels), cyclosporin A (an inhibitor of calcineurin), and PD08059 (an inhibitor of Erk ½) had no effect on Na+ and K+ content in EPS-treated C2C12 myotubes.
Cultured skeletal muscle cells subjected to electrical pulse stimulation (EPS) are widely employed as an in vitro model of exercising skeletal muscle [1–3]. This approach is based on several observations. First, in differentiated myotubes obtained from murine [4, 5] and rat [6] skeletal muscles (C2C12 and L6 cell lines, respectively) as well as in primary cultures of rat [7] and human [1] skeletal muscles, EPS resulted in metabolic and adaptive changes detected in freshly isolated trained muscles. Second, C2C12 myotubes exhibit remarkable contractile responses [4, 8,9] and repetitive [Ca2+]i transients [8] triggered by EPS. Third, during the last two decades it was shown that skeletal muscle functions as an exercise-dependent endocrine organ secreting dozens of myokines with molecular weights of 15-30 kDa (for review, see [10–12]). Several research groups reported that in cultured myotubes EPS triggers secretion of several exercise-dependent myokines [4, 5,13–16].
Numerous studies demonstrated that sustained excitation of skeletal muscle results in the dissipation of the transmembrane gradient of monovalent cations. Thus, in humans, intensive dynamic and static exercises lead to up-to 2-fold elevation of venous [K+] due to its release from skeletal muscle, i.e. a major source of intracellular K+ [17–20]). Calculation of femoral arterial-venous Na+ and K+ concentration differences based on the measurement of blood flow values, Na+ and K+ contents in muscle biopsies and changes of muscle water content demonstrated that in humans knee extensor intense exercises rapidly increase intracellular Na+ concentration ([Na+]i) in skeletal muscle from 13 to 23 mM and reduce [K+]i from 162 to 129 mM [21]. Using flame photometry, Murphy and coworkers found that in rat soleus muscle 60 min of intermittent running resulted in ~2-fold elevation in [Na+]i and attenuation of [K+]i by 10-20% [22].
Data considered above allowed us to propose that along with the well-documented impact of excitation-transcription coupling mediated by elevation of [Ca2+]i and activation of AMP-sensitive protein kinase (AMPK) and hypoxia-inducible factor HIF-1α [23], myokine production in exercising skeletal muscle might be triggered by the dissipation of transmembrane gradient of monovalent cations [12]. To examine this hypothesis, we compared the action of EPS on the intracellular concentration of Na+, K+, Ca2+, AMPK activity, and transcriptomic changes in C2C12 myotubes. We observed that similar to exercising skeletal muscle the transient EPS of C2C12 myotubes results in [Ca2+]i oscillations, dissipation of transmembrane gradients of monovalent cations and ~5-fold elevation in the Na+i/K+i ratio [16, 24]. We also found that inhibition of L-type voltage-gated Ca2+ channels by nicardipine abolished [Ca2+]i oscillations but did not affect the elevation of the Na+i/K+i ratio observed in EPS-treated myotubes [16]. Two-hr EPS resulted in differential expression of more than 3000 genes. Importantly, the differential expression of hundreds of EPS-sensitive genes was preserved in the presence of nicardipine. Among the nicardipine-resistant genes, we noted 113 genes whose expression was also affected by partial Na,K-ATPase inhibition by 30 mM ouabain providing the same elevation of the Na+i/K+i ratio as in EPS-treated cells. Unlike the elevation of the Na+i/K+i ratio and [Ca2+]i oscillation, EPS did not affect phosphorylation of acetyl-CoA carboxylase and Unc-51 like autophagy activating kinase-1, i.e. downstream markers of AMPK activation [16]. These results strongly suggested that elevation of the Na+i/K+i ratio affects gene expression in EPS-treated myotubes via Na+i/K+i-sensitive, Ca2+i-and AMPK-independent mechanism of excitation–transcription coupling.
During exercises, therefore, the impending loss of excitability has to be counterbalanced by rapid restoration of the Na+i/K+i ratio. To understand mechanisms of the maintenance of muscle contractility, it is necessary to know which transporters are participated in the dissipation of Na+i and K+i gradients and how to activate Na,K-ATPase for its regeneration.
This study was aimed at the identification of ion transporters involved in the dissipation of the transmembrane gradients of Na+ and K+induced by EPS, and Na,K-ATPase isoforms involved in its restoration.
Cell cultureMurine C2C12 cells (RRID: CVCL_0188) subjected to less than 10 passages were obtained from the Mammalian Cultures Collection of the Institute of Cytology of the Russian Academy of Sciences (Saint Petersburg, Russia). The cells were seeded at a density of 3×104 cells/well in 6-well plates containing Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 25 mM glucose, 10% heat-inactivated fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin and kept at 37°С in a humidified 5% CO2 atmosphere. Three-to-five days after plating, the cells reached 70-80% confluence and were subjected to differentiation in DMEM containing 25 mM glucose, antibiotics, 2% calf serum, and 1 nM insulin. The differentiation medium was changed daily. Cell morphology was evaluated by phase-contrast microscopy at ×100 magnification without preliminary fixation. Consistent with previous publications [4, 5,8, 9,13], we found that 7-day exposure to medium depleted of serum-derived growth factors and supplied with insulin resulted in myogenic differentiation of C2C12 cells indicated by cell fusion, formation of prolonged multinucleated myotubes, and appearance of troponin T [24]. The differentiated C2C12 myotubes were washed with phosphate buffered saline (PBS) and supplied with 3 ml of differentiation medium with addition of compounds listed in tables and figures legends. In some experiments, cells were subjected to EPS at 37oC for 2 hours using a C-Pace pulse generator (C-Pace EP, IonOptix, USA) with voltage 40 V, stimuli duration 10 ms, and frequency 1 Hz. Cell viability was assessed using the AlamarBlue assay [25] as described in detail elsewhere [24].
Rubidium influxTo examine the action of EPS on transmembrane monovalent cation fluxes, we employed Rb+ as analogue of K+ [26]. To study Rb+ influx, control and EPS-treated C2C12 myotubes were washed with PBS and incubated in shaking water-bath at 37oC in medium A containing 135 mM NaCl, 5 mM RbCl, 1 mM MgCl2, 1 mM CaCl2, 1 mM Na2HPO4, 10 mM glucose and 20 mM HEPES-tris (pH 7.4). In part of experiments, medium A contained inhibitors of ion transporters listed in figures legends. Fig. 1 displays kinetics of Rb+ accumulation in C2C12 myotubes. To study the rate of Rb+ influx, we limited incubation time to 10 min.
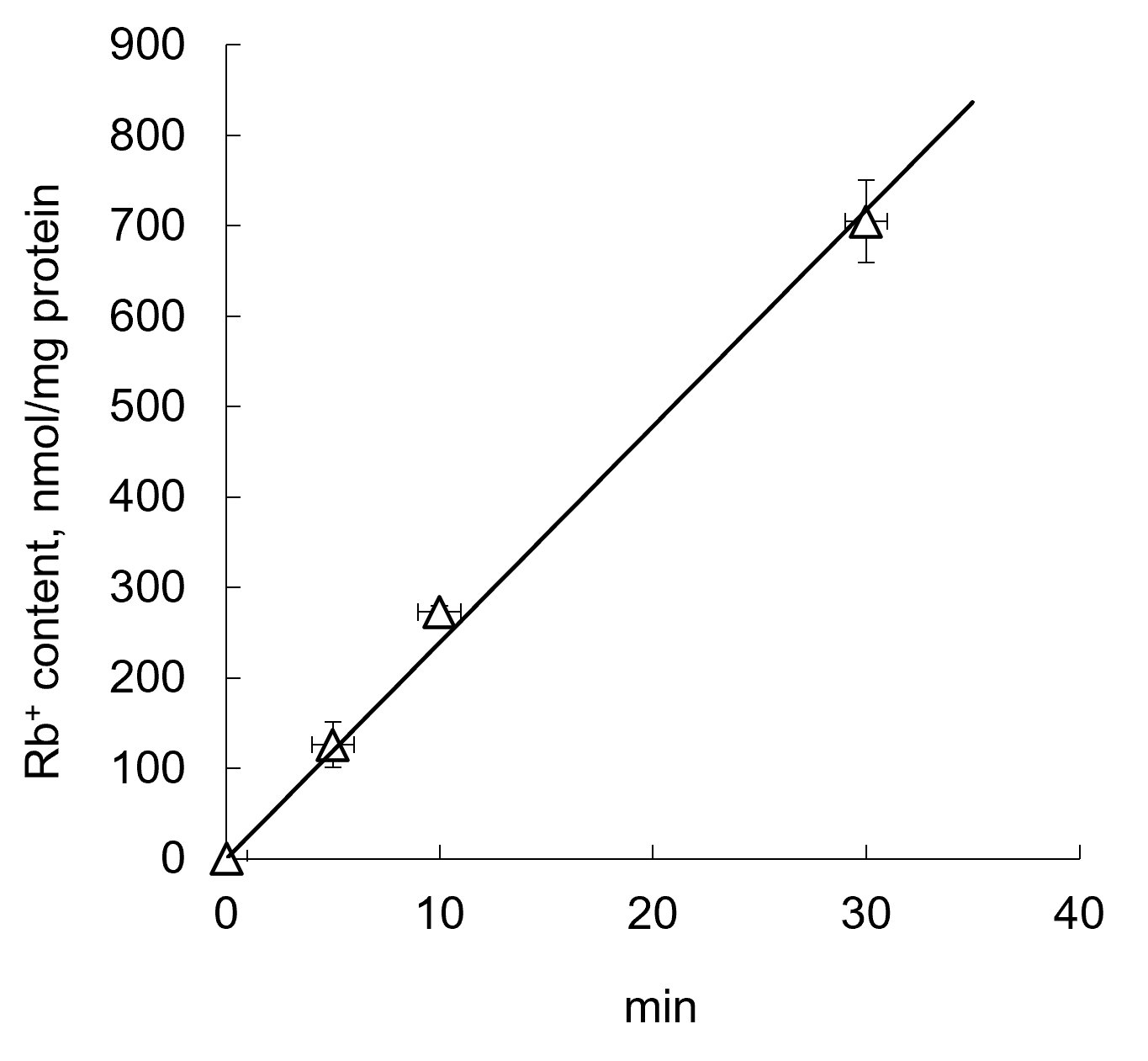
Figure 1. Kinetics of Rb+ influx by C2C12 myotubes. Rb+ uptake was measured in medium A containing 5 mM RbCl, respectively. Means ± S.E. obtained in 3 experiments are shown.
Measurement of intracellular Na+, K+, and Rb+ contentImmediately after electrical pulse stimulation, six-well plates were transferred onto ice, the experimental medium was quickly removed, and the cells were washed three times with 3 ml of ice-cold 0.1 M MgCl2 solution in doubly deionized (DDI) water. Then, 1.5 ml of 5% trichloroacetic acid (TCA) in DDI water was added to each well, followed by incubation at 4°С overnight for complete extraction of ions from myotubes. Cell precipitates were suspended and centrifuged for 5 min at 18, 000 relative centrifugal force. Supernatants were transferred to test tubes and stored at –20°С. Cell precipitates were resuspended in 0.75 ml of 0.1 M NaOH and incubated at 65°С for 1 h for complete protein dissolution. The Na+, K+, and Rb+ contents in TCA extracts were measured by flame atomic absorption spectrometry using a Kvant-2m1 spectrometer (Cortec, Russia) with propane-air mixture in accordance with the manual at slit of 0.25 mm and wavelengths of 766.5 nm (K+), 589 nm (Na+), 780 nm (Rb+). Solutions of KCl (0.5-4 mg/liter K+), NaCl (0.05-2 mg/liter Na+), and RbCl (0.2-4 mg/liter Rb+) in 5% TCA in DDI water were used for calibration. The ion contents in each well were normalized to protein amount in the same well, which was determined by Lowry protein assay [27].
MaterialsDMEM was purchased from both PanEco (Russia) and Gibco (USA). Trypsin-EDTA was obtained from PanEco (Russia), and penicillin-streptomycin, calf serum, and heat-inactivated fetal bovine serum were purchased from Gibco (USA). Cell culture equipment was from Corning (USA). AlamarBlue reagent was obtained from Invitrogen (USA). Insulin and ouabain were from Sigma-Aldrich (USA); MgCl2 from Fluka Analytical (Switzerland); trichloroacetic acid (TCA) from Fisher BioReagents (USA). Bumetanide, amiloride, 5-(N-Ethyl-N-isopropyl) amiloride (EIPA), furosemide, cyclosporin A was purchased from Sigma-Aldrich (USA); apamin from GenScript Biotech (USA); charybdotoxin from BaChem (Switzerland); glibenclamide from TCI (Japan); noxiustoxin from Alomone Labs (Israel); KN-62, nicardipine hydrochloride from ABCR (Germany); KN-93 from TargetMol Chemicals Inc. (USA); PD98059 from Calbiochem (USA). Unless otherwise noted, all chemicals were of the purest grade available from Sigma-Aldrich (USA), Fischer BioReagents (USA), Fluka Analytical (Switzerland), Amresco (USA) or ThermoFisher (USA).
Statistical analysis
All data are expressed as means ± standard error and analyzed by Dunnett's multiple comparison OneWay ANOVA test. The threshold for significance was p < 0.05.
α2-Na,K-ATPase provides the maintenance of Na+i/K+i gradient in EPS-treated C2C12 myotubesNa,K-ATPase comprises α- and β-subunits, which together form a functional αβ-heterodimer [28]. Major Na,K-ATPase α-subunit isoforms expressed in skeletal muscle [29] and C2C12 cells [30] are α1- and α2-subunits. In contrast to number of other cell types studied, both C2C12 cells [30] and skeletal muscle (for review, see [31, 32]) are abundant with α2-isoform of Na,K-ATPase whose content is 3-5-fold higher compared to housekeeping α1-isoform. Importantly, in mice and several other rodents the affinity of α1-isoform for inhibitory action of ouabain is 3 order of magnitude less compared to α2-Na,K-ATPase [33]. Thus, using NIH 3T3 fibroblasts transfected with cDNAs encoding distinct α-subunits O’Brien and co-workers demonstrated that the apparent affinity of rat α2- and α1-Na,K-ATPase for ouabain calculated from [3H]-ouabain binding and ATPase assay yielded values of ~115 and 48, 000 nM, respectively [34]. In mouse and rat skeletal muscle, 10 mM ouabain completely inhibits α2-Na,K-АТPase without significant effect on α1-Na,K-АТPase whose full-scale inhibition was observed in the presence of 1000-3000 mM ouabain [35]. We used this feature of mice-derived C2C12 cell line to examine the relative impact of α1- and α2-isozymes in the regulation of intracellular Na+i and K+i content in control and EPS-treated myotubes.
Consistently with previous reports [16, 24], we observed that 2-hr EPS resulted in the 2-fold elevation of Na+i and attenuation of K+iintracellular content (Table 1). In accordance with results obtained in rat skeletal muscle strips [36], the complete inhibition of Na,K-АТPase by 3000 mM ouabain led to the sharp elevation of the Na+i/K+i ratio and abolished the action of EPS. At the concentration of 10 mM, ouabain did not affect the baseline content of monovalent cations but increased the increment of the Na+i/K+i ratio evoked by EPS. These results suggest that EPS increases the relative impact of α2-containing Na,K-АТPase in the maintenance of transmembrane Na+i and K+i gradients.
Indeed, Fig. 2 shows that the activity of α2-containing Na,K-АТPase measured as a component of the rate of Rb+ influx inhibited by 10 mM ouabain was increased in EPS-treated myotubes from 1.9±2.1 up-to 7.3±2.4 nmol/mg protein min. In contrast to low doses of ouabain, the full-scale inhibition of Na,K-АТPase by 3000 mM ouabain resulted in ~3-fold attenuation of Rb+ influx in both control and EPS-treated myotubes (Fig. 2).
We suggest that the stronger effect of the Na,K-АТPase containing α2-subunit on the maintenance of Na+i and K+i gradient in EPS-treated C2C12 myotubes is associated not only with the high abundance of this isoform but also with its activation. It was demonstrated that in intact mouse fibers the apparent affinities of α1- and α2-Na,K-АТPase for extracellular K+ are 1.3 and 4 mM, respectively [37]. It is known also that Na+-sensitivity of α1- and α2-Na,K-АТPase is the same [38]. These data and the measurement of K+o-dependent regulation of the electrical membrane potential in isolated muscle fibers allowed to propose that in exercising skeletal muscle α2-Na,K-АТPase activity is stimulated by an increment of K+o concentration in the T-tubule limited space [39].
We may also suggest that the increase of [Na+]i/[K+]i ratio during EPS may result in the elevation of the α2-subunit expression. It was shown that physical exercises induce an increase in α2-subunit Na,K-АТPase expression in skeletal muscle [40], and the modulation of Na+ transport into cells also triggers α2-isoform-specific expression in C2C12 [30]. The physiological significance of α2-subunit of Na,K-АТPase in contracting muscle is confirmed by the data of Radzyukevich and coworkers who developed a gene-targeted ska2-/- mouse in which Na,K-АТPase containing α2-subunit is completely absent in the transverse tubules of skeletal muscle cells, where its expression in wild-type animals is normally enhanced compared to housekeeping α1-isoform [41]. They reported that ska2-/- mice are normal under baseline conditions but exhibit reduced exercise capacity when forced to run.
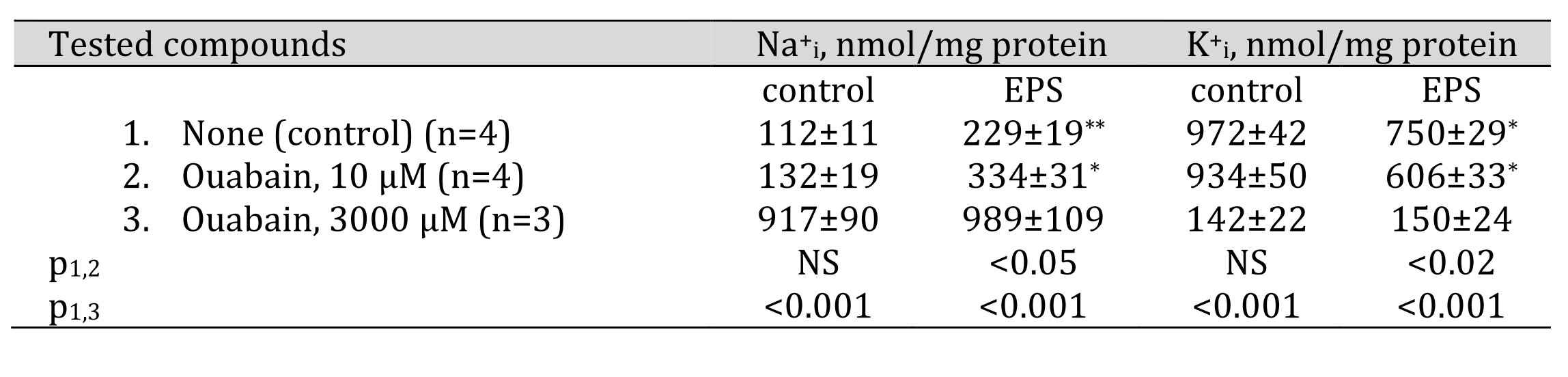
Table 1. Effect of ouabain on intracellular sodium and potassium content in control and EPS-treated C2C12 myotubes. C2C12 myotubes were supplied with 3 ml of differentiation medium containing compounds listed in the left column. In some experiments, cells were subjected to EPS for 2 hours with voltage 40 V/10 ms/1 Hz. Means ± S.E. obtained in n experiments are shown. *, ** p<<0.01 and 0.002 compared to control (EPS-untreated) cells
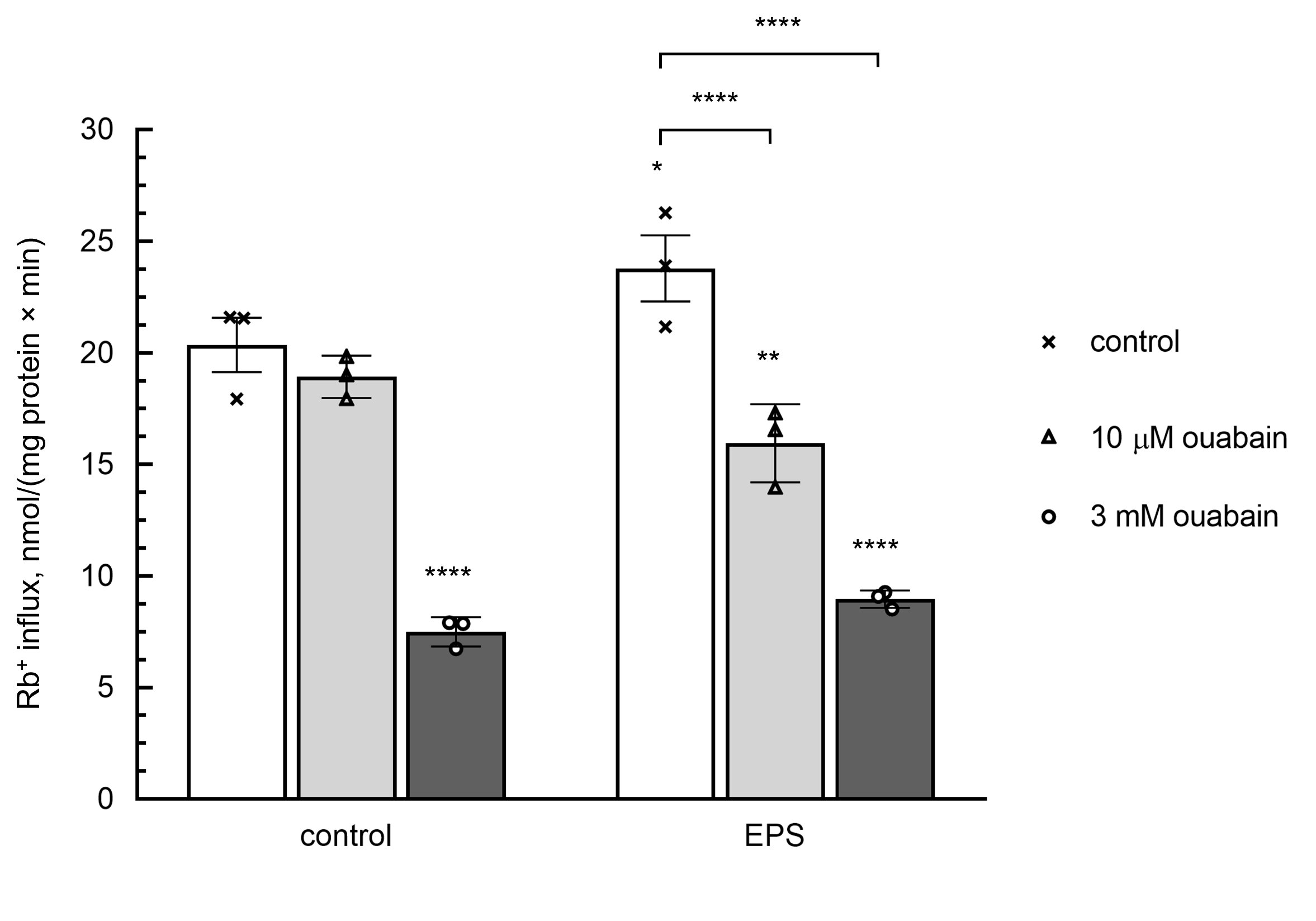
Figure 2. Effect of ouabain on the rate of Rb+ influx in C2C12 myotubes. In part of experiments, cells were subjected to EPS as indicated in Table 1 legend. Then, control and EPS-treated myotubes were supplied with medium A containing RbCl and with or without 10 or 3000 μM ouabain and incubated during 10 min in the presence or absence of EPS. Means ± S.E. obtained in 3 experiments are shown.
Voltage-gated Na+ (Nav), and large conductance Ca2+-activated K+ channels (BKCa), and Na/H exchanger (NHE) are responsible for Na+i/K+i gradient dissipation in EPS-treated C2C12 myotubesIt is known that sarcolemma and T-tubule of skeletal muscle are abundant with Nav1.4, Nav1.5, Nav1.6 voltage-gated Na+ channels, Kv1.4, Kv3.4, Kv7.5 voltage-gated K+ channels as well as with large conductance (KCa1.1) and small conductance (KCa2.2 and KCa2.3) Ca2+-activated K+ channels [42, 43]. Simon et al. demonstrated the presence of α-, β- and γ-subunits of epithelial sodium channels (ENaC) in rat muscle spindles [44]. Their expression and activity in resting and EPS-treated C2C12 myotubes have not yet been investigated.
Table 2 shows that the gain of Na+i seen in EPS-treated myotubes was completely abolished by 0.5 mM tetrodotoxin, an inhibitor of voltage-gated Na+ channel, and by 10 mM EIPA, an inhibitor of Na/H exchanger, whereas the loss of K+i was suppressed by 0.1 mM charybdotoxin, an inhibitor of large conductance Ca2+-activated K+ channels. Neither the inhibitor of voltage-gated Kv1.2 and Kv1.3 K+channels noxiustoxin, nor the inhibitor of ATP-sensitive K+ channels glibenclamide affected the loss of K+i triggered by EPS. We also did not detect any significant impact of inhibitors of intermediate and small conductance Ca2+-activated K+ channels (TRAM-34 and apamin, respectively) on this parameter. None of the tested compounds affected the intracellular content of Na+i and K+i in control EPS-untreated myotubes. The data clearly demonstrate that the dissipation of transmembrane Na+i and K+i gradient observed in EPS-treated C2C12 myotubes is mainly associated with the activation of tetrodotoxin-sensitive voltage-gated Na+ channels (Nav), Na/H exchanger (NHE) and charybdotoxin-sensitive large conductance Ca2+-activated K+ channels (BKCa). Our results do not contradict the previously published data. Thus, voltage-gated Na+ channels and large conductance Ca2+-activated K+ channels are necessary to maintain the excitability of skeletal muscle cells [45, 46]. At the same time, changes in skeletal muscle cells pH during exercise are observed, and Na/H exchanger is participated in intracellular pH recovery following it [47].
In epithelial cells, 1 mM amiloride almost completely suppresses ENaC activity (for review, see [48]). Unlike epithelial cells, in skeletal muscle Na+ channels triggered by stretch were slightly inhibited by 1 mM amiloride but completely blocked after the addition of 1000 mM amiloride [44]. Table 2 shows that both the elevation of [Na+]i and the attenuation of [K+]i triggered by EPS were resistant to 1 mM amiloride but sharply diminished in the presence of 1000 mM amiloride indicating the presence of channels with low affinity for this compound. A direct effect of 1000 mM amiloride on NHE is also possible, since such an effect has been observed previously in chick muscle cells [49]. However, it should be emphasized that high amiloride concentrations can also inhibit the activity of diverse protein kinase involved in intracellular signaling cascades, side-by-side with ENaC and NHE [50]. It is worth noting that 1000 mM amiloride also eliminates EPS-mediated K+efflux (Table 2). However, there is only one mention in the literature of amiloride-sensitive K+channels identified in rabbit renal brush border membrane [51]. It has also been shown that c wave produced by K+ currents in rabbit electroretinogram reduced by 100 mM amiloride [52].
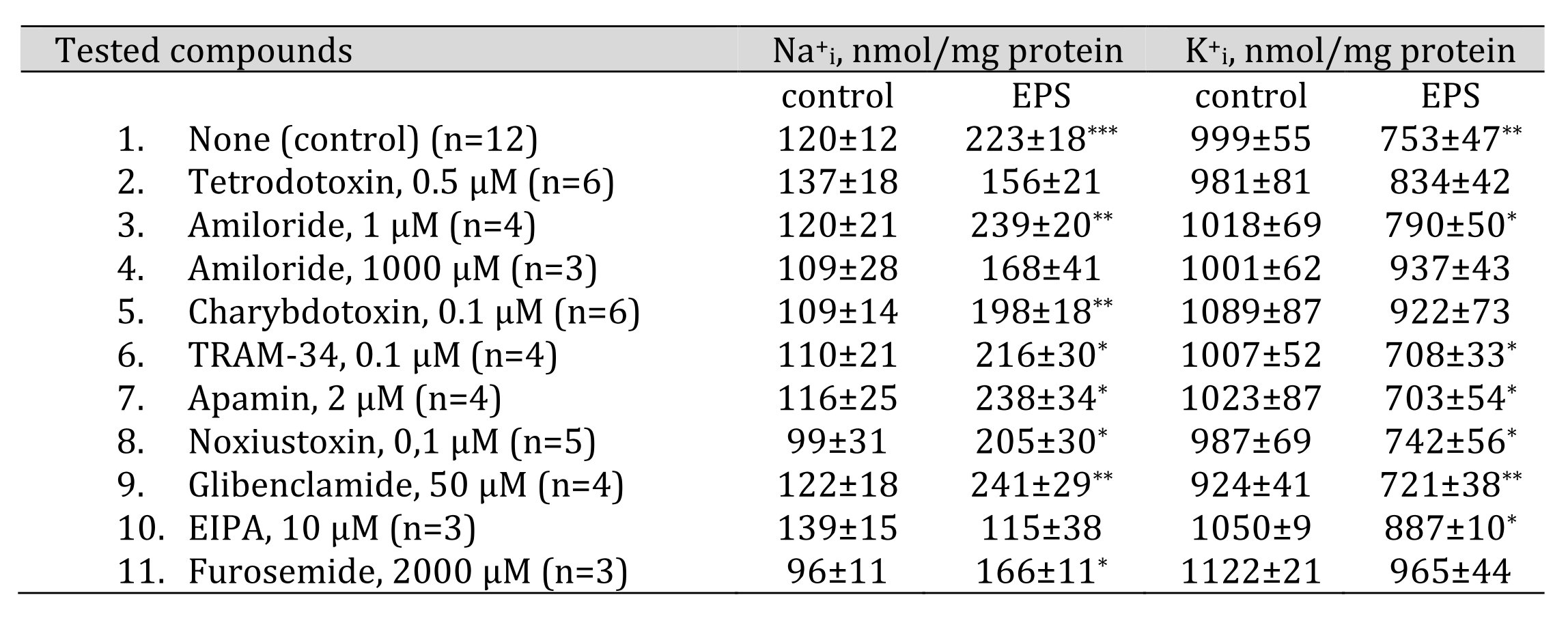
Table 2. Effects of ion transport inhibitors on intracellular sodium and potassium content in control and EPS-treated C2C12 myotubes. C2C12 myotubes were supplied with 3 ml of differentiation medium containing compounds listed in the left column. In some experiments, cells were subjected to EPS with voltage 40 V/10 ms/1 Hz. Means ± S.E. obtained in n experiments are shown. *, **, *** p<0.05, <0.01 and <0.001 compared to control (EPS-untreated) cells
EPS increases Na,K,2Cl cotransporter activity in C2C12 myotubesNa-Cl cotransporter (NCC), Na-K-2Cl cotransporters (NKCC), and K-Cl cotransporters (KCC) belong to the family of solute carriers involved in the transport of ions across biological membranes along or against their electrochemical gradient. It is known that NCC and NKCC are inhibited by low doses of bumetanide and thiazide derivatives, respectively. There are two potent specific inhibitors of КСС(VU0255011 and VU0463271), and their activity is known to be partially attenuated in the presence of high doses of furosemide [53].
Immunoreactive NKCC1 was detected in both red and white skeletal muscle as well as in rat skeletal muscle-derived immortalized L6 myoblasts [54]. Two isoforms of KCCs (KCC1 and KCC3) have been identified in skeletal muscles (for review, see [55, 56]). In L6 myoblasts, bumetanide inhibited Rb+ uptake by 50-60% [54, 57] thus indicating the highly active NKCC. Unlike L6 myoblasts, bumetanide did not affect Rb+ uptake in freshly isolated rat soleus and plantaris skeletal muscles [58]. CCC activity in C2C12 myotubes has not been examined yet. To understand which of these transporters are involved in the dissipation of Na+i and K+i gradient, we performed an inhibitor analysis.
Fig. 3 shows that EPS increased NKCC activity measured as ouabain-resistant, bumetanide-sensitive component of the rate of Rb+influx (1.7±0.4 and 2.8±0.5 nmol/mg protein min in control and EPS-treated myotubes, respectively). In the presence of bumetanide, addition of 2000 mM furosemide did not affect the rate of Rb+ uptake thus indicating a negligible activity of inwardly directed KCC. Neither bumetanide nor furosemide affected the intracellular Na+i and K+i content under baseline conditions, as well as the increment of the Na+i/K+i ratio triggered by EPS (Table 3). All data together demonstrate that in C2C12 myotubes EPS appears to increase the activity of Na-K-2Cl cotransporter providing K+ influx. On the other hand, when interpreting these data, it should be kept in mind that the analysis of ion fluxes was performed after the end of electrical pulse stimulation and not during it. This fact does not exclude that NKCC may be activated to restore gradients of monovalent cations.
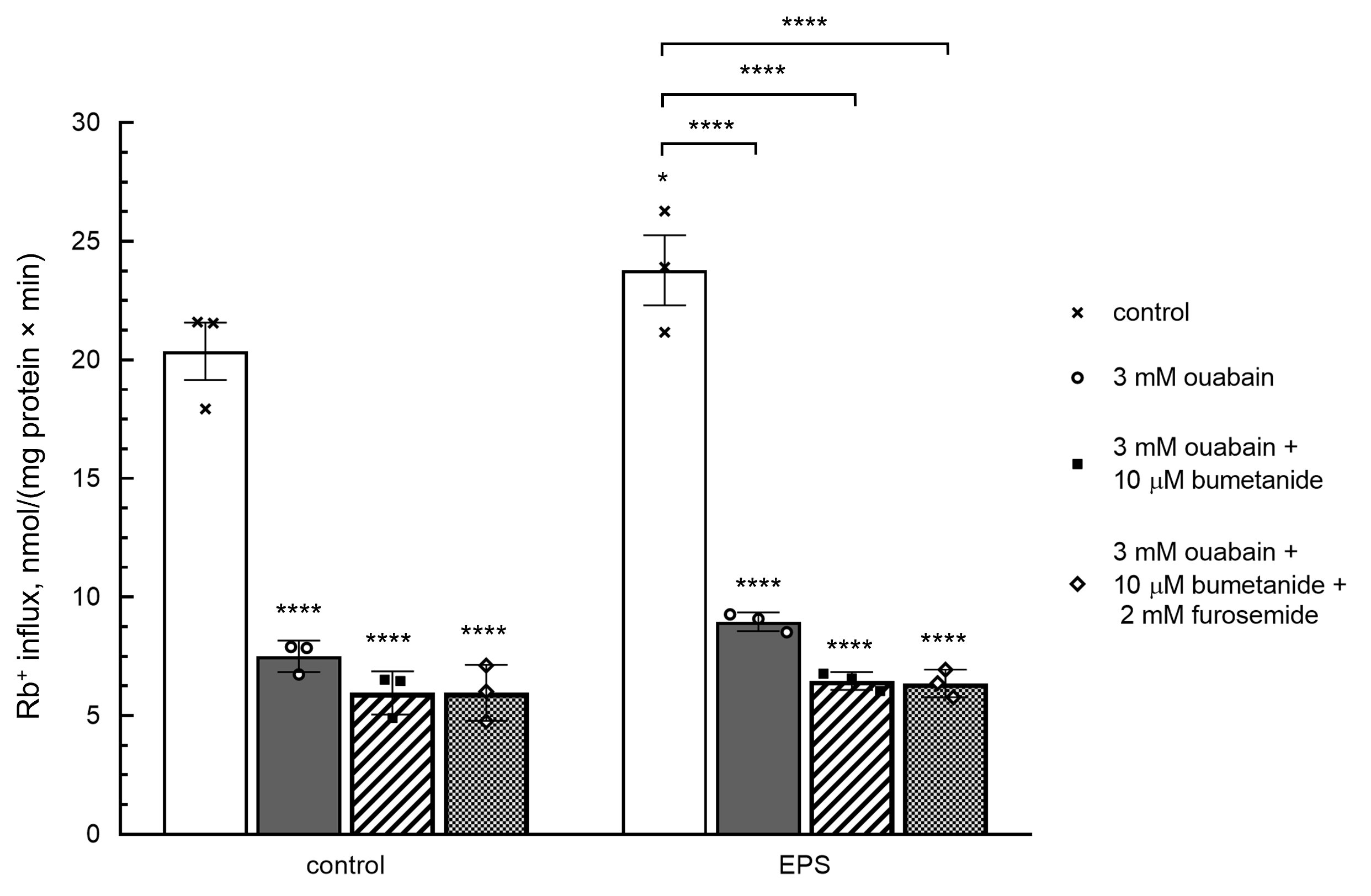
Figure 3. Effect of bumetanide and furosemide on the rate of Rb+ influx in C2C12 myotubes. In part of experiments, cells were subjected to EPS as indicated in Table 3 legend. Then, control and EPS-treated myotubes were supplied with medium A containing RbCl, 3000 μM ouabain and with or without 20 μM bumetanide and 2000 μM furosemide and incubated during 10 min in the presence or absence of EPS. Means ± S.E. obtained in 3 experiments are shown.
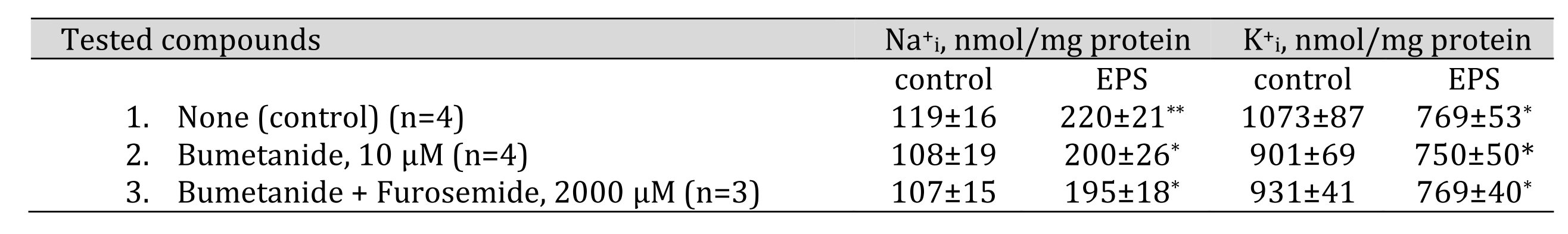
Table 3. Effect of bumetanide and furosemide on intracellular sodium and potassium content in control and EPS-treated C2C12 myotubes. C2C12 myotubes were supplied with 3 ml of differentiation medium containing compounds listed in the left column. In some experiments, cells were subjected to EPS with voltage 40 V/10 ms/1 Hz. Means ± S.E. obtained in n experiments are shown. *, ** p<0.05 and <0.01 compared to control (EPS-untreated) cells
Suppression of calmodulin-dependent protein kinase II (CaMKII) by KN-62 and KN-93 increases Na+ efflux in EPS-treated C2C12 myotubesIn order to identify elements of EPS-activated signaling system(s), we also used an inhibitor assay. Table 4 shows that inhibition of calmodulin-dependent protein kinase II (CaMKII) activity by 10 mM KN-62 or 10 mM KN-93 results in reduced Na+i content in EPS-treated myotubes compared with control EPS-treated samples. At the same time, 10 mM KN-93 also decreased Na+i content in EPS-untreated C2C12 myotubes. Previously, 5 mM KN-93 was shown to reduce Na+ currents in rat ventricular myocytes [59]. It is possible that this effect of KN-93 is nonspecific, unrelated to CaMKII activity [60]. The effect of these inhibitors was diminished but did not disappear completely after the inhibition of Na,K-ATPase by ouabain. Apparently, CaMKII suppresses Na,K-ATPase activity and/or affects some other Na+ transporters which leads to the activation of Na+ influx or to the inhibition of Na+ efflux. It is most likely that CaMKII inhibition decreases the activity of the Na/H exchanger, which contributes to the gain of Na+i in EPS-treated C2C12 myotubes (Table 3). This observation is supported by data obtained using rat cardiomyocytes that CaMKII activates the Na/H exchanger in these cells in order to recover intracellular pH [61].
We did not detect any significant action of 10 mM nicardipine (an inhibitor of L-type voltage-gated Ca2+ channels), 10 mMcyclosporin A (an inhibitor of calcineurin), 10 and 40 mM PD98059 (an inhibitor of Erk ½) on intracellular content of monovalent cations in control C2C12 myotubes as well as in EPS-treated samples.
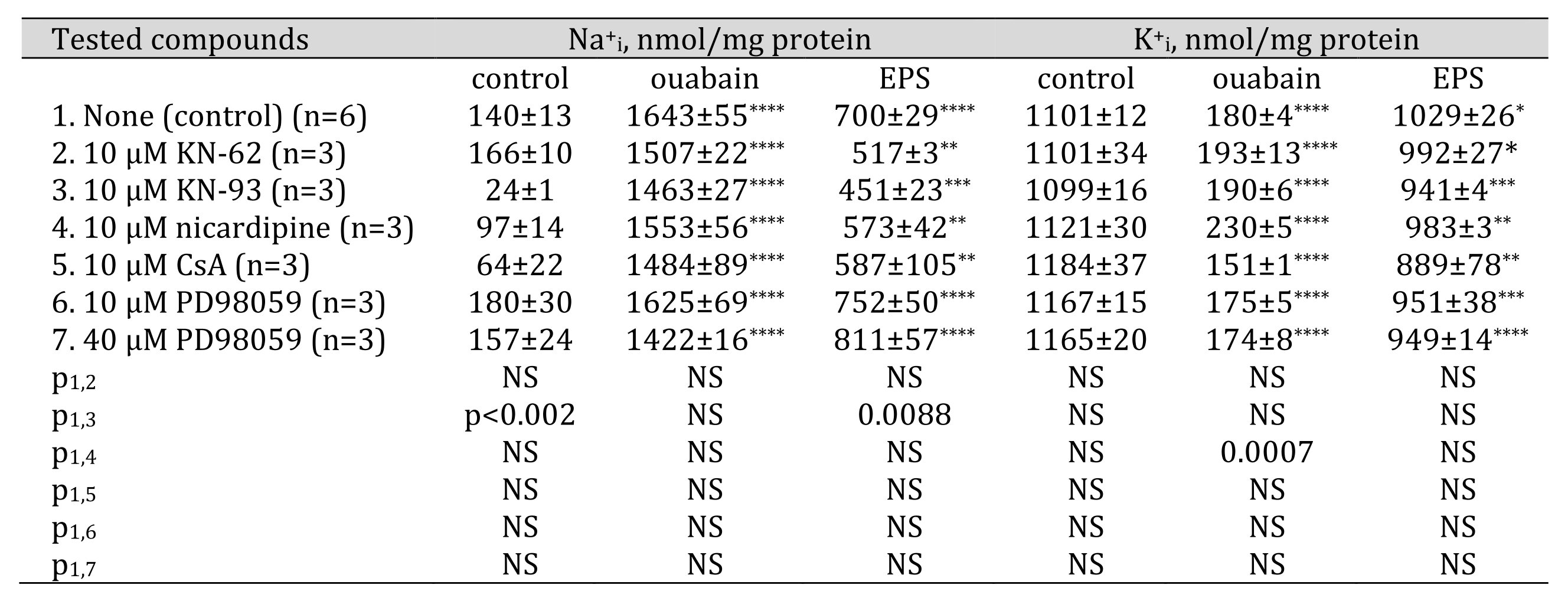
Table 4. Effect of Ca2+-activated signaling systems inhibitors on intracellular sodium and potassium content in control, 3000 M ouabain and EPS-treated C2C12 myotubes. C2C12 myotubes were supplied with 3 ml of differentiation medium containing compounds listed in the left column. In some experiments, cells were subjected to EPS with voltage 40 V/10 ms/1 Hz. Means ± S.E. obtained in n experiments are shown. *, **, ***, **** 0.0189, 0.0038, 0.0009 and <0.0001 compared to control (ouabain and EPS-untreated) cells
The data obtained in the present studies led us to five major conclusions.
First, the dissipation of transmembrane Na+i and K+i gradients observed in EPS-treated C2C12 myotubes is mainly caused by the activation of tetrodotoxin-sensitive voltage-gated Na+ channels (Nav), Na/H exchanger (NHE) and charybdotoxin-sensitive Ca2+-activated large-conductance K+ channels (BKCa).
Second, EPS is accompanied by the activation of α2-Na,K-АТPase that partially suppresses the dissipation of transmembrane Na+iand K+i gradients.
Third, in C2C12 myotubes, EPS appears to increase the activity of Na-K-2Cl cotransporters measured as ouabain-resistant bumetanide-sensitive component of Rb+ influx.
Fourth, we did not find any evidence that voltage-gated Kv1.2 and Kv1.3 K+ channels, as well as ATP-sensitive K+ channels and small conductance Ca2+-activated K+ channels affect EPS-induced K+i loss in C2C12 cells.
Fifth, calmodulin-dependent protein kinase II appears to stimulate Na+ influx in EPS-treated C2C12 myotubes.The impact of Na+ and K+ fluxes trough different transporters in the dissipation of Na+i and K+i gradients is the subject of further studies, as well as the role of various protein kinase that are inhibited by high amiloride concentrations.
Dedicated to the memory of Alexey A. Vereninov.We express our gratitude to the Interdisciplinary Scientific and Educational School of Moscow University «Molecular Technologies of the Living Systems and Synthetic Biology».
Author contributions
Svetlana V. Sidorenko: Investigation, Software, Visualization; Elizaveta A. Klimanova: Data curation, Writing - Original draft preparation, Reviewing and Editing; Olga D. Lopina: Writing - Original draft preparation, Reviewing and Editing; Eugene G. Maksimov: Writing - Reviewing and Editing; Sergei N. Orlov: Conceptualization, Methodology, Supervision, Writing - Original draft preparation.
Funding SourcesThis work was supported by a grant from the Russian Scientific Foundation RSF #16-15-10026-п.
Statement of Ethics
The authors have no ethical conflicts to disclose.
The authors have no conflicts of interest to declare.