

Review - DOI:10.33594/000000704
Accepted 23 April, 2024 - Published online : 20 May, 2024
1Institute of General Practice and Public Health, Claudiana – College of Health Professions, 39100 Bolzano, Italy,
2Department of Public Health, Medical Decision Making and HTA, University of Health Sciences, Medical Informatics and Technology, 6060 Hall in Tyrol, Austria
Background/Aims: This review examines the therapeutic potential of recombinant human albumin compared to that of plasma-derived human serum albumin in critical conditions, such as cirrhosis and sepsis, highlighting its safety, efficacy, pharmacokinetics and pharmacodynamics. Methods: A narrative review was conducted, evaluating existing literature from databases including PubMed, EMBASE, and ClinicalTrials.gov to explore the clinical applications and therapeutic potential of human serum albumin and its recombinant forms. Results: The review emphasizes the consistent quality and reduced heterogeneity of recombinant human albumin. Based on current evidence from preclinical and clinical research, recombinant human albumin and plasma-derived human serum albumin show comparable colloid osmotic pressure and pleiotropic activities, with the potential superiority of recombinant human albumin in ligand-binding properties. Despite promising studies in man, challenges to cost-effective production and research gaps remain. Conclusions: Further studies are needed to define the clinical utility of recombinant human albumin in established and promising indications for plasma-derived serum albumin in cirrhotic, critically ill and perioperative patients.
Human serum albumin (HSA) plays a major role in clinical practice, mainly because of its ability to maintain colloid osmotic pressure in the plasma blood vessels and its ability to act as a carrier for a wide range of substances. This protein is essential for maintaining intravascular volume and facilitating the transport of hormones, drugs, and other molecules throughout the body [1]. Its importance extends beyond these physiological roles, as HSA solutions derived from plasma also have therapeutic applications in various medical conditions, where administration of HSA is linked to managing fluid balance, supporting hemodynamic stability, and reducing inflammation particularly in states of hypoalbuminemia [2, 3].
The proven use of plasma-derived HSA in critically ill patients is based on its documented therapeutic benefits, particularly in conditions such as hypoalbuminemia associated with acute kidney injury, where plasma-derived HSA administration has shown preventive capabilities in specific scenarios such as cirrhosis with ascites and spontaneous bacterial peritonitis [4]. In patients with liver cirrhosis, HSA is recommended for specific subgroups, highlighting its critical role in managing complications, such as hepatorenal syndrome and large-volume paracentesis, thereby positively affecting survival in targeted populations [5]. In addition, plasma-derived HSA has demonstrated efficacy in enhancing diuretic response, particularly in hypoalbuminemic patients, thereby facilitating fluid management in the acute setting. This extends to improving fluid removal during kidney replacement therapy, highlighting its utility in managing fluid overload and preventing intradialytic hypotension in patients with acute kidney injury or end-stage kidney disease [4]. The role of plasma-derived HSA in sepsis or septic shock is still under investigation, with studies suggesting its potential benefits in hemodynamic stabilization and improved survival in selected patient cohorts [4, 6]. This breadth of application not only underlines the therapeutic importance of plasma-derived HSA in the management of critically ill patients but also highlights the ongoing exploration of its full potential across a spectrum of clinical conditions [7].
Recombinant human albumin (rHA) represents a significant advancement in biotechnological and pharmaceutical applications, providing a safe and consistent alternative to plasma-derived HSA. Its role as a stabilizer in the formulation of therapeutic products, including vaccines, biologics, and drug-delivery systems, underscores its utility in enhancing stability and improving yield [8, 9]. Beyond its pharmaceutical applications, rHA is essential in ex vivo contexts, such as cell culture systems, where it supports cell growth and viability, and in vitro fertilization, where it improves the medium for embryo development [10–12]. For example, to investigate the relationship between proteinuria and the progression of kidney disease by examining the effect of HSA on kidney cell growth, opossum kidney proximal tubular cells were assayed for their effects on cell proliferation. Recombinant HA promoted cell proliferation in a dose-dependent manner by activating ERK, and was successfully inhibited by PD98059, an inhibitor of p44/p42 extracellular-signal-regulated mitogen-activated protein kinase (ERK-MAP) pathway. These effects were specific to rHA, as neither ovalbumin nor mannitol replicated these results, suggesting that HSA interaction with kidney cells involves the signaling pathway and can be induced by rHA preparations [13]. This versatility not only highlights the critical function of rHA in biotechnological practices but also opens avenues for its potential therapeutic applications, including intravenous rHA therapy in cirrhosis, critically ill and perioperative patients, pending further clinical development and research. Traditionally sourced from human plasma, concerns over the supply and pathogen transmission of plasma-derived HSA have stimulated rHA production using technologies such as bacterial and yeast expression systems. Despite progress, achieving cost-effective large-scale production that meets safety standards remains a significant challenge [14].
The objective of this narrative review is to evaluate the potential of rHA in the clinical management of conditions where plasma-derived HSA has proven beneficial or promising, particularly in cirrhosis and sepsis, and to contrast it with plasma-derived HSA. The literature search was expanded beyond PubMed to include EMBASE and ClinicalTrials.gov, utilizing ‘recombinant human albumin’ as the search term. By examining the characteristics and comparative effects of rHA in both preclinical and clinical studies, this review aims to provide an assessment of its future potential in these patient populations, highlighting innovative approaches and gaps in current knowledge.
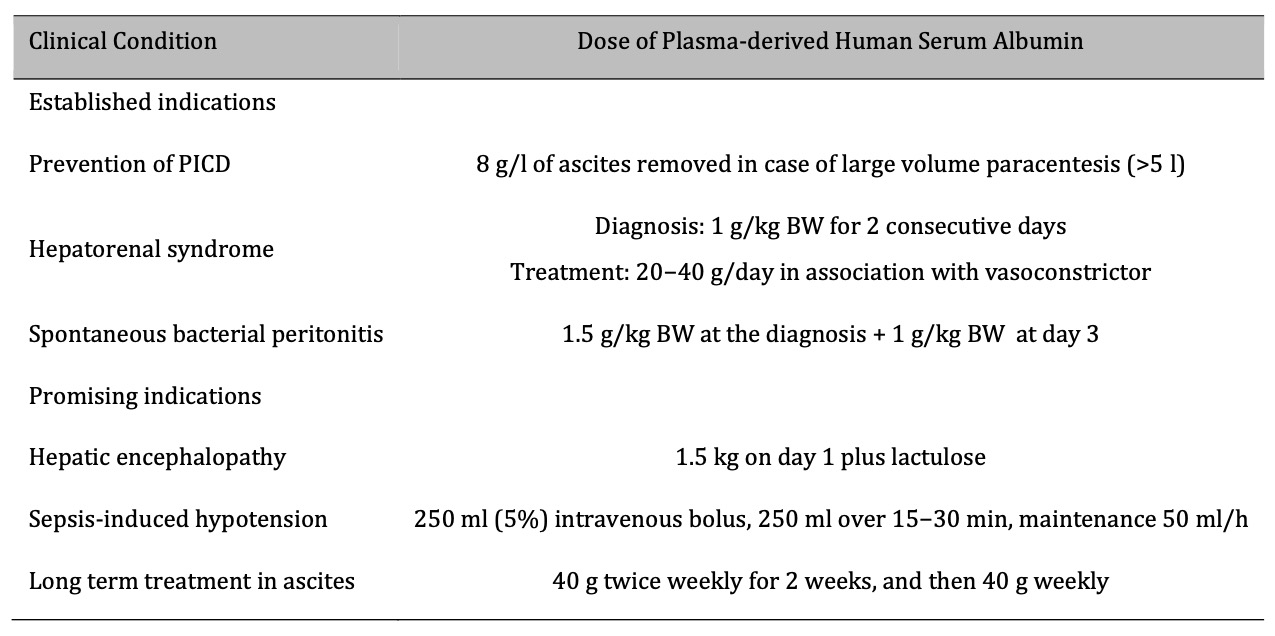
Table 1. Established and promising indications for use of plasma-derived human serum albumin in cirrhosis. PICD: paracentesis induced circulatory dysfunction; BW, body weight. Reproduced under a CC BY-NC-ND 4.0 License from Fundación Clínica Médica Sur, A.C. Published by Elsevier España, S.L.U. (accessed on February 5, 2024) Copyright © 2023, This reuse has not been endorsed by the licensor. The source reference is “[15]” and is available at: https://doi.org/10.1016/j.aohep.2023.101150 1665-2681/ (accessed on 5 February 2024)
Cirrhosis leads to significant changes in the body's fluid balance and vascular function owing to liver dysfunction [15]. The role of albumin in maintaining colloid osmotic pressure is critical in the management of ascites and peripheral edema, which are common complications of cirrhosis. The pathophysiological basis is its ability to retain fluid in the circulatory system, thereby reducing the tendency of fluid to accumulate in the peritoneal cavity and tissues. The anti-inflammatory effect of albumin plays a critical role in mitigating renal complications and myocardial dysfunction associated with cirrhosis [16, 17]. Altered or deficient endogenous albumin in cirrhosis, often due to molecular modifications, compromises its protective effects, including the binding of harmful substances. The administration of high doses of plasma-derived HSA can compensate for this deficiency, providing a therapeutic strategy to restore the functional capacity of albumin, thereby preventing the progression of renal complications and improving cardiac function in patients with cirrhosis [18].
In sepsis, vascular permeability increases, leading to the leakage of albumin and other plasma proteins into the interstitial space, which can lead to edema and contribute to hypovolemia. Albumin supplementation in sepsis aims to restore vascular volume, support endothelial barrier function, and modulate inflammatory response (Fig. 1). This rationale includes the antioxidant properties of albumin and its role in binding and neutralizing toxins and inflammatory mediators [7, 15].
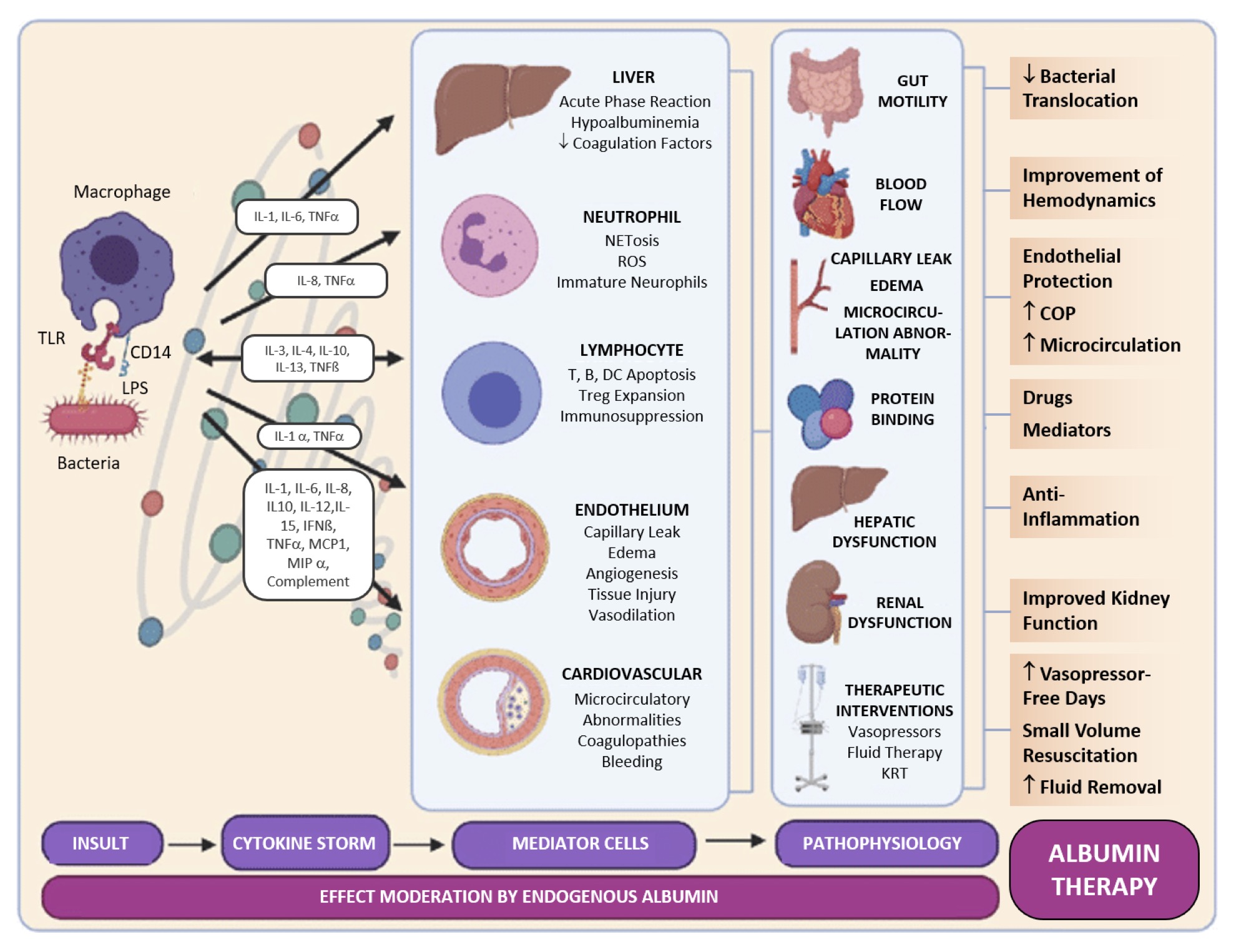
Figure 1. Sepsis pathophysiology and human serum albumin therapy. TLR: toll-like receptor; LPS: lipopolysaccharide; IL: interleukin; TNF: tumor necrosis factor; IFN: interferon; MCP: monocyte chemoattractant protein; MIP: macrophage inflammatory protein; NET: neutrophil extracellular trap; ROS: reactive oxygen species; DC: dentritic cell; Treg: regulatory T cell; KRT: kidney replacement therapy. Reproduced under a CC BY-NC-ND 4.0 License with modifications from: Sanz Codina and Zeitlinger [35] (accessed on February 5, 2024), under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) License (https://creativecommons.org/licenses/by-nc-nd/4.0/ (February 5, 2024). Copyright © 2022, The Authors. This reuse has not been endorsed by the licensor. The source reference is “[35]” and is available at https://link.springer.com/article/10.1007/s40262-021-01102-1 (accessed on 5 February 2024).
The pathophysiological rationale for albumin supplementation in both conditions includes multiple roles, beyond volume expansion. These include their effects on improving microcirculation, stabilizing endothelial cell barriers, and their potential immunomodulatory effects [7]. In cirrhosis, albumin helps to manage hyperdynamic circulation, while in sepsis, it addresses capillary leak syndrome and supports organ function. Understanding the pathophysiological mechanisms underlying the therapeutic use of albumin in cirrhosis and sepsis underscores the importance of targeted therapy. This suggests that the benefits of albumin extend beyond simple volume expansion to include the modulation of inflammatory responses and improvement of endothelial function, which are critical in the management of these complex conditions.
The current utilization of plasma-derived HSA in clinical settings extends to various controversial indications such as hypoalbuminemia, ischemic brain injury, extraperitoneal infections in cirrhosis, and sepsis, particularly in scenarios without shock and/or severe hypoalbuminemia. Despite its theoretical benefits, the application of plasma-derived HSA under these conditions is debated owing to inconsistent evidence regarding its efficacy and safety [2]. The expected oncotic and pleiotropic properties of HSA may not consistently translate into tangible clinical outcomes due to several factors, including albumin leakage from the intravascular compartment, compensatory role of other plasma proteins, potential adverse effects, and changes during production and storage [14, 19].
The theoretical benefits of rHA over plasma-derived HSA focus on its profile, which is devoid of modifications inherent to plasma-derived HSA production. Recombinant HA produced in Saccharomyces cerevisiae and plasma-derived HSA were structurally compared, revealing equivalent conformations. Analytical assessments showed that the rHA version exhibited reduced structural heterogeneity compared with its blood-derived counterpart, suggesting a more uniform molecular composition in the recombinant product [20]. This consistency arises from controlled manufacturing processes that eliminate the variability and contamination risks associated with plasma-derived HSA, such as oxidation and alterations in binding capacity [14]. The absence of these modifications in rHA potentially enhances its antioxidant capacity and drug and metabolite binding efficacy, and reduces pro-inflammatory actions, offering a purer and more predictable therapeutic option.
Changes in the manufacturing process of plasma-derived HSA, such as the introduction of stabilizers during pasteurization, can impair its ability to bind and transport substances. Additionally, variations in the redox capacity of plasma-derived HSA across different products suggest that manufacturing techniques can differentially alter the tertiary structure of HSA, affecting its functional integrity and therapeutic potential [14]. Modifications of plasma-derived HSA during production and storage can alter its pleiotropic properties, potentially affecting its effectiveness. These modifications may impair albumin binding, detoxification, and antioxidant capabilities, raising concerns about the clinical implications of using modified albumin, especially in conditions that require its pleiotropic non-oncotic functions [19].
The manufacturing process, including heat treatment, can generate neoantigens, potentially affecting the immunogenicity of albumin products [21]. However, current evidence does not suggest that these manufacturing-related alterations significantly impact the immunogenicity of albumin for clinical use [14]. The differential adverse effects between rHA and plasma-derived HSA are likely minimal and do not significantly affect their respective indications. Both forms of albumin have been shown to possess similar safety profiles when used as stabilizers, making their therapeutic use broadly comparable [22]. The choice between recombinant and plasma-derived HSA may instead be guided by factors such as availability, cost, and specific clinical requirements, rather than concerns over adverse effects.
Recombinant HA is now produced using recombinant DNA technology, overcoming the limitations of plasma-derived HSA, such as supply and quality inconsistencies [23]. Pichia pastoris yeast is a leading source of rHA, enabling large-scale, high-quality output. Techniques include genetic manipulation to enhance post-translational modifications and reduce the number of purification steps. Additionally, plant seed bioreactors, such as those using Oryza sativa seeds, offer alternative methods that produce rHA structurally and functionally equivalent to their plasma-derived counterparts. The functional equivalence of plasma-derived HSA and rHA from transgenic rice seeds was tested through structural, biochemical, and efficacy studies [24]. Molecular comparisons showed identical molecular masses, amino acid sequences, and crystal structures between the recombinant and plasma-derived HSA. Functional tests involved binding capacity assessments with specific drug markers and promotion of cell growth in vitro, which demonstrated comparable affinities and cell growth promotion between rHA and plasma-derived HSA. Additionally, in vivo studies in rats for treating liver cirrhosis indicated similar therapeutic efficacy between rHA and plasma-derived HSA [24].
Both plasma-derived HSA and rHA exhibited similar anti-inflammatory activity. This is evidenced by their ability to modulate leukocyte responses, particularly by inhibiting cytokine expression induced by CpG-DNA, a common component of bacterial and mitochondrial DNA where a cytosine nucleotide occurs next to a guanine nucleotide in the linear sequence of bases along its length [25]. These findings suggest that the anti-inflammatory mechanisms of albumin, whether rHA or plasma-derived HSA, effectively intervene in inflammatory signaling pathways within leukocytes, offering a basis for their therapeutic use in conditions characterized by inflammation. Another study comparing plasma-derived HSA and rHA found that rHA exhibited superior thermal stability and enhanced cell growth activity in vitro [26]. This was attributed to differences in the binding of lipid mediators, suggesting that the recombinant HA structure could be more favorable for certain biological functions, potentially impacting its therapeutic applications.
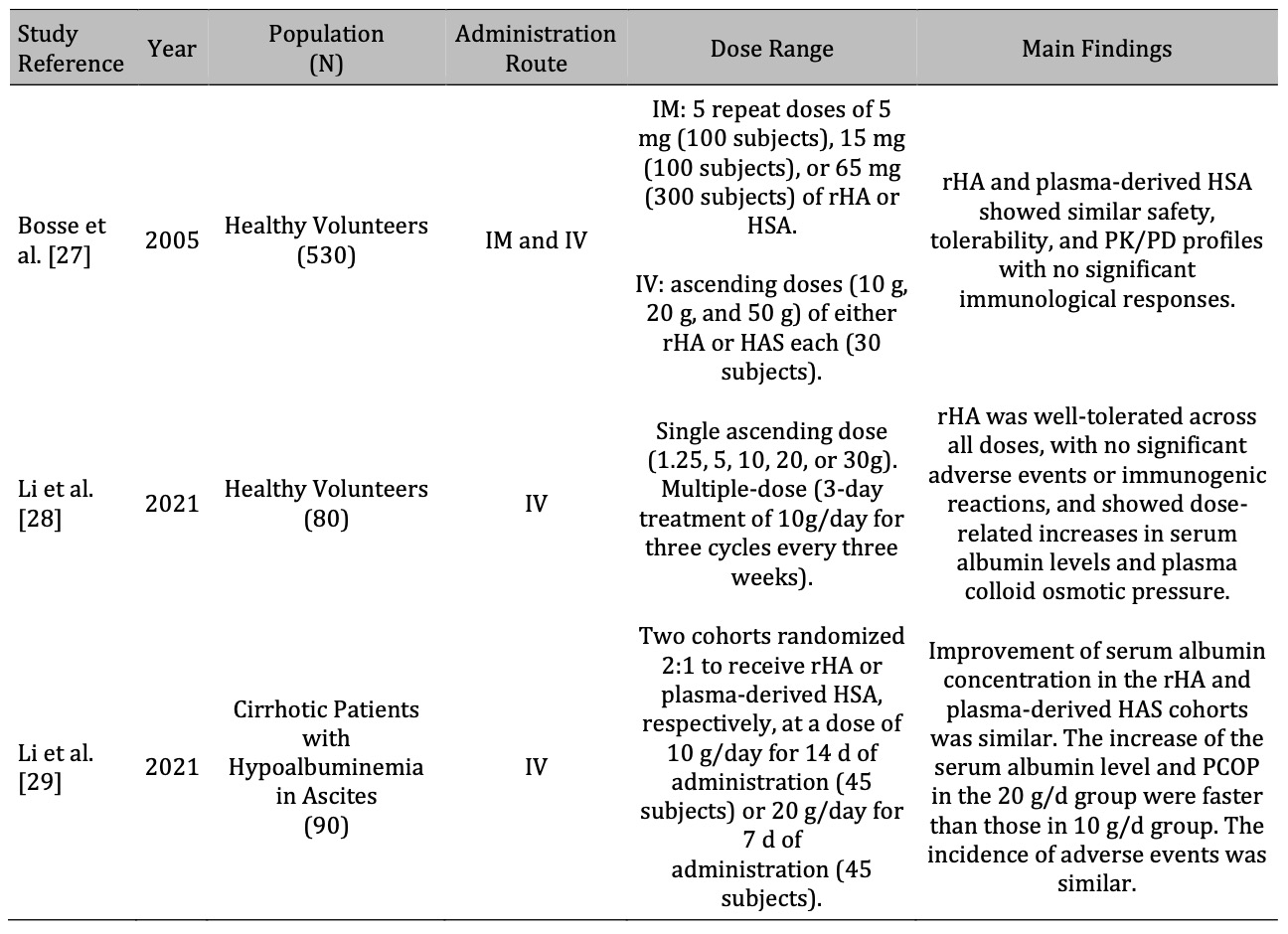
Table 2. Summary of early-phase clinical studies on recombinant human albumin. rHA: recombinant human albumin; IM: intramuscular; IV: intravenous; PK/PD: pharmacokinetics/pharmacodynamics; HAS: human serum albumin; PCOP: plasma colloid osmotic pressure
Table 2 summarizes the available evidence from early-phase clinical studies on rHA, highlighting its safety, tolerability, pharmacokinetics and pharmacodynamcis, and efficacy compared with plasma-derived HSA. Given the nascent stage of clinical research, the data presented are still limited but provide insights for future development plans of phase III trials that are underway.
A phase I study by Bosse et al. [27] compared rHA and plasma-derived HSA regarding safety, tolerability, and pharmacokinetics/pharmacodynamics in healthy volunteers. It included two double-blind randomized trials, one using intramuscular and another using intravenous administration in 500 and 30 volunteers, respectively. Serum albumin, colloid osmotic pressure changes, and the hematocrit ratio were as expected, with no differences between rHA and plasma-derived HSA. The study found that rHA and plasma-derived HSA exhibited similar safety, tolerability, pharmacokinetic, and pharmacodynamic profiles, with no significant immunological responses to either product [27]. These results suggest the comparability between rHA and plasma-derived HSA for clinical use.
Li et al. [28] evaluated the safety, tolerability, immunogenicity, and pharmacokinetics and pharmacodynamics (PK/PD) of different rHA products in healthy volunteers. This was a double-blind, first-in-human study in healthy subjects aged 18-55 years comparing single ascending doses of rHA (ranging from 1.25 to 30 g) and a positive-controlled multiple-dose regimen (10 g daily for three cycles every three weeks) with placebo. The safety evaluation of the study focused on adverse events, whereas immunogenicity was assessed by analyzing antibodies (immunoglobulins IgE and IgD) and cytokines. The PK/PD effects were assessed based on serum albumin levels, plasma colloid osmotic pressure, and hematocrit changes. The results showed that rHA was well tolerated at all doses, with all adverse events classified as mild or moderate. There was no significant difference in the incidence of adverse events between dose groups in the single ascending-dose study or across cycles in the multiple-dose study, and the incidence was comparable between the rHA and plasma-derived HSA cohorts. Additionally, no significant changes in antibodies or cytokines were observed after drug administration. Dose-related increases in serum albumin levels, plasma colloid osmotic pressure, and decreases in hematocrit were observed, demonstrating significant effects, with no notable differences between rHA and plasma-derived HSA [28].
In a subsequent phase Ib study, the same authors evaluated the safety, tolerability, PK/PD, and efficacy of rHA in cirrhotic patients with ascites compared to those of plasma-derived HAS [29]. Thirty-six patients at three medical centers were randomized to receive up to 14 days of treatment with rHA or plasma-derived HSA at various doses (10 g, 20 g, and 30 g) and followed for 28 days. Adverse events, serum albumin levels, plasma colloid osmotic pressure changes, ascites resolution, abdominal circumference, and weight changes were also evaluated. The study showed that rHA was well tolerated at all doses, with no significant difference in the incidence of adverse events between the groups or dose-related adverse events. PK/PD analysis showed similar trends in serum albumin concentration and changes in plasma colloid osmotic pressure in both the groups. Efficacy analyses showed improvements in ascites, abdominal circumference, and body weight in most subjects, without a clear dose effect. Immunogenicity studies have not shown positive results [29]. The conclusion is that rHA is safe and has similar tolerability, pharmacokinetics/pharmacodynamics, and efficacy profiles to HSA, supporting further large-scale phase II trials to evaluate its efficacy and safety in cirrhotic patients with ascites.
Reported as poster at the ‘European Association for the Study of the Liver’ (EASL) Congress in 2023 and reported as an abstract [30], the Indian ‘Al-Fit study’ in cirrhotic patients aimed to evaluate the efficacy of a combination therapy consisting of weekly rHA infusions (100 ml of 20% solution), personalized nutritional counseling, and home-based physiotherapy in improving physical performance, reducing decompensation events, and enhancing quality of life. Randomizing 60 patients with ‘model for end-stage liver disease’ (MELD) scores ≥ 15 into two groups, the study found significant improvements in the ‘Liver Frailty Index’, MELD score, and quality of life scores after 3 months in the treatment group compared to standard care [30]. However, no information on the type of rHA is given in the abstract and the ‘Clinical Trial Registry India’ (CTRI) number provided in the abstract could not be verified on February 2, 2024, highlighting the importance of ensuring trial registration details are accurate and verifiable for research transparency and validation. Further assessment of the evidence base for rHA awaits full publication of the study.
Building on the approved indications for plasma-derived HSA, research on rHA aims to open up new therapeutic perspectives, improve patient outcomes, and establish the role of rHA in critical care. For liver-related indications, rHA is currently in the early stages of clinical development, with some trials recently completed and partially reported and others actively underway.
In a recruiting randomized, double-blind, placebo-controlled phase II/III clinical trial evaluating the effectiveness and safety of rHSA versus plasma-derived HSA in patients with hepatic cirrhosis and ascites in China, the impact of 10 or 20 g/day for 14 or 7 days of rHA, respectively, on serum albumin levels, dose-efficacy relationship, safety profile, pharmacodynamic characteristics, and immunogenicity was assessed. Participants included adults with decompensated cirrhosis and ascites in parallel groups receiving different doses and treatment durations of rHSA or plasma-derived HSA, focusing on serum albumin concentration changes, ascites depth changes, abdominal circumference, and body weight after follow-up until day 57 [31].
While research on the treatment of cirrhosis and ascites is progressing, there is less public awareness of the benefits of plasma-derived HSA for other established or controversial indications. Future research should focus on addressing these gaps, possibly through clinical trials designed to rigorously evaluate the efficacy, safety, and pharmacokinetics of rHA in a wider range of conditions. These studies could focus on patient populations where plasma-derived HSA is currently used and investigate whether rHA offers comparable or superior outcomes, thereby supporting its wider use in medical practice.
The annual global demand for HSA surpassed 500 tons in 2013 and was traditionally met through plasma extraction [32]. The limitations of plasma sources and contamination risks have led to the development of recombinant DNA technologies for rHA production using various hosts. Addressing the biopharmaceutical manufacturing of rHA, it is critical to recognize the unique challenges posed by the need for production at a much larger scale than most other recombinant proteins. The requirement to produce rHA in kilogram to tonne quantities surpasses current capabilities of standard recombinant protein production technologies. This limitation restricts the use of rHA primarily to niche medical indications or potentially as excipients in other medicinal products. The latter use has been complicated by historical issues related to infectious risks, such as variant Creutzfeldt-Jakob disease, associated with plasma-derived HA. These production challenges highlight the need for innovative manufacturing solutions to enable the broader use of rHA in clinical practice and underscore the importance of continued research and development in this area.
The economics of rHA production must also be considered in the context of the current market dynamics for plasma-derived proteins. HSA, often a by-product of immunoglobulin production by plasma fractionation, benefits from cost absorption strategies that significantly reduce its price. This economic model poses a challenge to the cost-effectiveness of producing rHA independently. As immunoglobulins are cost-effective therapies and drive the economics of plasma fractionation, rHA production requires innovative approaches to reduce costs to be competitive. Despite advancements, cost-effective and safe large-scale production of rHA that meets stringent quality standards remains a challenge, highlighting the need for efficient production strategies [14]. To make rHA a viable alternative to plasma-derived HSA, it is essential to explore potential strategies for reducing production costs [33]. This could include optimizing biotechnological processes, increasing yields through genetic engineering, and exploiting economies of scale for production. By focusing on these areas, the aim was to reduce the price of rHA, making it accessible and economically competitive, thereby expanding its potential for therapeutic applications.
Regulatory approval processes pose a significant hurdle, requiring comprehensive data on safety, efficacy, and manufacturing consistency. Additionally, market acceptance depends on demonstrating clear advantages of rHA over plasma-derived HSA, such as improved safety profiles or cost benefits. Strategic partnerships of clinical scientists with biotechnology firms will accelerate development and facilitate market entry. Finally, ongoing research to uncover new therapeutic applications of rHA could expand its clinical utility, making it a more attractive option for healthcare providers and patients alike.
The successful integration of rHA into clinical practice could have a significant impact on healthcare beyond the treatment of patients with cirrhosis and sepsis [34]. This opens the possibility of treating a wide range of conditions in which the volume expansion, drug binding, and antioxidant properties of albumin are beneficial. This could lead to improved patient outcomes, reduced dependence on blood-derived products, and improved safety profiles by minimizing the risks associated with blood-borne pathogens. In addition, it could stimulate further research into novel therapeutic applications and precision medicine strategies, ultimately expanding the range of available treatments for various medical conditions.
This narrative review underscores the critical role of HSA in clinical practice, highlighting its physiological and therapeutic importance in the management of fluid balance, hemodynamic stability, and inflammatory state in various medical conditions. Recombinant HA is a promising, safe, and consistent alternative to plasma-derived HSA, with potential applications extending beyond pharmaceutical stabilization to therapeutic use in critically ill patients. This discussion highlights the need for further clinical development and research to explore the full potential of rHA, particularly in cirrhosis and sepsis, where its comparative efficacy and safety have yet to be fully elucidated. Future perspectives should focus on reducing production costs to make rHA a viable option, potentially revolutionizing patient care by expanding therapeutic applications and improving accessibility.
As the sole author of this paper, I conducted research and manuscript preparation independently, utilizing ChatGPT, Deepl Write, and PaperPal as digital tools to assist in drafting the article.
The authors received fees from CSL Behring (speaking and consulting), Grifols, and Biotest (speaking).